Androgen receptor pathway inhibitors and taxanes in metastatic prostate cancer: an outcome-adaptive randomized platform trial
- PMID: 39164518
- PMCID: PMC11564108
- DOI: 10.1038/s41591-024-03204-2
Androgen receptor pathway inhibitors and taxanes in metastatic prostate cancer: an outcome-adaptive randomized platform trial
Erratum in
-
Author Correction: Androgen receptor pathway inhibitors and taxanes in metastatic prostate cancer: an outcome-adaptive randomized platform trial.Nat Med. 2024 Nov;30(11):3381. doi: 10.1038/s41591-024-03268-0. Nat Med. 2024. PMID: 39198712 Free PMC article. No abstract available.
Abstract
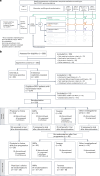
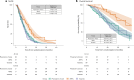
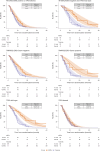
ProBio is the first outcome-adaptive platform trial in prostate cancer utilizing a Bayesian framework to evaluate efficacy within predefined biomarker signatures across systemic treatments. Prospective circulating tumor DNA and germline DNA analysis was performed in patients with metastatic castration-resistant prostate cancer before randomization to androgen receptor pathway inhibitors (ARPIs), taxanes or a physician's choice control arm. The primary endpoint was the time to no longer clinically benefitting (NLCB). Secondary endpoints included overall survival and (serious) adverse events. Upon reaching the time to NLCB, patients could be re-randomized. The primary endpoint was met after 218 randomizations. ARPIs demonstrated ~50% longer time to NLCB compared to taxanes (median, 11.1 versus 6.9 months) and the physician's choice arm (median, 11.1 versus 7.4 months) in the biomarker-unselected or 'all' patient population. ARPIs demonstrated longer overall survival (median, 38.7 versus 21.7 and 21.8 months for taxanes and physician's choice, respectively). Biomarker signature findings suggest that the largest increase in time to NLCB was observed in AR (single-nucleotide variant/genomic structural rearrangement)-negative and TP53 wild-type patients and TMPRSS2-ERG fusion-positive patients, whereas no difference between ARPIs and taxanes was observed in TP53-altered patients. In summary, ARPIs outperform taxanes and physician's choice treatment in patients with metastatic castration-resistant prostate cancer with detectable circulating tumor DNA. ClinicalTrials.gov registration: NCT03903835 .
© 2024. The Author(s).
Conflict of interest statement
Figures
References
-
- van der Velden, D. L. et al. The Drug Rediscovery protocol facilitates the expanded use of existing anticancer drugs. Nature574, 127–131 (2019). - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- STI.VLK.2020.0006.01/Vlaamse Liga Tegen Kanker (Flemish Cancer League)
- STI.VLK.2022.0005.01/Vlaamse Liga Tegen Kanker (Flemish Cancer League)
- KLbB-5580-02-2022/Krebsliga Beider Basel (Cancer League of Basel-City and Basel-Country)
- 21 1610 P/Cancerfonden (Swedish Cancer Society)
- 2021-00331/Vetenskapsrådet (Swedish Research Council)
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

