Engineered mitochondria exert potent antitumor immunity as a cancer vaccine platform
- PMID: 39164536
- PMCID: PMC11528120
- DOI: 10.1038/s41423-024-01203-4
Engineered mitochondria exert potent antitumor immunity as a cancer vaccine platform
Abstract
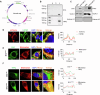
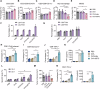
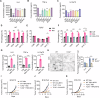
The preferable antigen delivery profile accompanied by sufficient adjuvants favors vaccine efficiency. Mitochondria, which feature prokaryotic characteristics and contain various damage-associated molecular patterns (DAMPs), are easily taken up by phagocytes and simultaneously activate innate immunity. In the current study, we established a mitochondria engineering platform for generating antigen-enriched mitochondria as cancer vaccine. Ovalbumin (OVA) and tyrosinase-related protein 2 (TRP2) were used as model antigens to synthesize fusion proteins with mitochondria-localized signal peptides. The lentiviral infection system was then employed to produce mitochondrial vaccines containing either OVA or TRP2. Engineered OVA- and TRP2-containing mitochondria (OVA-MITO and TRP2-MITO) were extracted and evaluated as potential cancer vaccines. Impressively, the engineered mitochondria vaccine demonstrated efficient antitumor effects when used as both prophylactic and therapeutic vaccines in murine tumor models. Mechanistically, OVA-MITO and TRP2-MITO potently recruited and activated dendritic cells (DCs) and induced a tumor-specific cell-mediated immunity. Moreover, DC activation by mitochondria vaccine critically involves TLR2 pathway and its lipid agonist, namely, cardiolipin derived from the mitochondrial membrane. The results demonstrated that engineered mitochondria are natively well-orchestrated carriers full of immune stimulants for antigen delivery, which could preferably target local dendritic cells and exert strong adaptive cellular immunity. This proof-of-concept study established a universal platform for vaccine construction with engineered mitochondria bearing alterable antigens for cancers as well as other diseases.
Keywords: Antitumor immunity; Cardiolipin; Mitochondria vaccine; TLR2.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

