Opicapone for the treatment of early wearing-off in levodopa-treated Parkinson's disease: pooled analysis of patient level data from two randomized open-label studies
- PMID: 39164557
- PMCID: PMC11447072
- DOI: 10.1007/s00415-024-12614-8
Opicapone for the treatment of early wearing-off in levodopa-treated Parkinson's disease: pooled analysis of patient level data from two randomized open-label studies
Erratum in
-
Correction: Opicapone for the treatment of early wearing-off in levodopa-treated Parkinson's disease: pooled analysis of patient level data from two randomized open-label studies.J Neurol. 2025 Aug 1;272(8):549. doi: 10.1007/s00415-025-13122-z. J Neurol. 2025. PMID: 40748463 Free PMC article. No abstract available.
Abstract
Background: The wearing-off phenomenon is a key driver of medication change for patients with Parkinson's disease (PD) treated with levodopa. Common first-line options include increasing the levodopa dose or adding a catechol-O-methyltransferase (COMT) inhibitor, but there are no trials comparing the efficacy of these approaches. We evaluated the effectiveness of adjunct opicapone versus an additional 100 mg levodopa dose in PD patients with early wearing-off using pooled data from 2 randomized studies.
Methods: The ADOPTION study program included two similarly designed 4-week, open-label studies conducted in South Korea (NCT04821687) and Europe (NCT04990284). Patients with PD, treated with 3-4 daily doses of levodopa therapy and with signs of early wearing-off were randomized (1:1) to adjunct opicapone 50 mg or an additional dose of levodopa 100 mg. Patient-level data from the two studies were pooled.
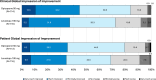
Results: The adjusted mean [SE] change from baseline to week 4 in absolute OFF time (key endpoint) was - 62.8 min [8.8] in the opicapone group and - 33.8 min [9.0] in the levodopa 100 mg group, the difference significantly favoring opicapone (- 29.0 [- 53.8, - 4.2] min, p = 0.02). Significant differences in the Movement Disorder Society-Unified Parkinson's Disease Rating Scale Part III subscore (- 4.1 with opicapone vs - 2.5 with levodopa 100 mg), also favored opicapone (- 1.7 [- 3.3, - 0.04], p < 0.05). Dyskinesia was the most frequently reported adverse event (opicapone 7.2% vs. levodopa 100 mg 4.2%).
Conclusions: In these short-term trials, introducing adjunct opicapone was more effective at reducing OFF time than adding another 100 mg levodopa dose in PD patients with early signs of wearing-off.
Keywords: COMT; Levodopa; Motor fluctuations; Opicapone; Parkinson’s disease; Wearing-off.
© 2024. The Author(s).
Conflict of interest statement
Joaquim J. Ferreira has provided consultancy and received speaker fees from Lundbeck, BIAL, Biogen, Acadia, Abbvie, Sunovion Pharmaceuticals, Zambon, Affiris, Roche, ONO and SK Chemicals and has received grants from Abbvie, Bial, Medtronic and Angelini. Jee-Young Lee received a research grant of NRF funded by the MSIT of Korea and a multidisciplinary research grant-in-aid and a focused clinical research grant-in-aid from the Seoul Metropolitan Government-Seoul National University (SMG-SNU) Boramae Medical Center, and speaker honorarium from Esai Korea, Bial, SK chemicals and Scientific Advisory Board of Regeners Inc. Beomseok Jeon has received research grant from Peptron and Abbvie Korea and has participated in an expert panel for Aspen Neuroscience. Werner Poewe has received lecture fees and honoraria for consultancy in relation to clinical drug development programs from Alterity, AbbVie, Affiris, AstraZeneca, Axovant, BIAL, Biogen, Britannia, Lilly, Lundbeck, NeuroDerm, Neurocrine, Denali Pharmaceuticals, Orion Pharma, Roche, Stada, Sunovion, Takeda, UCB and Zambon, as well as grant support from the MJFF and the EU FP7 & Horizon 2020 programs. Angelo Antonini has received compensation for consultancy and speaker-related activities from UCB, Boehringer Ingelheim, Britannia, AbbVie, Zambon, Bial, NeuroDerm, Theravance Biopharma, Roche; he receives research support from Chiesi Pharmaceuticals, Lundbeck, Horizon 2020 - Grant 825785, Horizon2020 Grant 101016902, Ministry of Education University and Research (MIUR) Grant ARS01_01081, Cariparo Foundation. He serves as consultant for Boehringer Ingelheim for legal cases on pathological gambling; owns Patent WO2015110261-A1; and owns shares in PD Neurotechnology Limited. Fabrizio Stocchi has received compensation for consultancy and speaker related activities from Lundbeck, UCB, Chiesi, Zambon, Britannia, Cynapsus, Sunovion, Kyowa, Abbvie, NeuroDerm, Biogen, Bial. Daniela M. Rodrigues, Miguel M. Fonseca, Guillermo Castilla-Fernández, Joerg Holenz and José-Francisco Rocha are employed by BIAL. Olivier Rascol has participated in advisory boards and/or provided consultancy for AbbVie, Adamas, Acorda, Addex, AlzProtect, ApoPharma, AstraZeneca, Axovant, Bial, Biogen, Britannia, Buckwang, CereSpir, Clevexel, Denali, INC Research, IPMDS, Lundbeck, Lupin, Merck, MundiPharma, NeurATRIS, NeuroDerm, Novartis, ONO Pharma, Osmotica, Parexel, Pfizer, Prexton Therapeutics, Quintiles, Roche, Sanofi, Servier, Sunovion, Theranexus, Takeda, Teva, UCB, Vectura, Watermark Research, XenoPort, XO, Zambon; received grants from Agence Nationale de la Recherche (ANR), CHU de Toulouse, France-Parkinson, INSERM-DHOS Recherche Clinique Translationnelle, MJ Fox Foundation, Programme Hospitalier de Recherche Clinique, European Commission (FP7, H2020), Cure Parkinson UK; and received a grant to participate in a symposium and contribute to the review of an article by the International Parkinson and Movement Disorder Society.
Figures
References
-
- Pringsheim T, Day GS, Smith DB, Rae-Grant A, Licking N, Armstrong MJ, de Bie RMA, Roze E, Miyasaki JM, Hauser RA, Espay AJ, Martello JP, Gurwell JA, Billinghurst L, Sullivan K, Fitts MS, Cothros N, Hall DA, Rafferty M, Hagerbrant L, Hastings T, O’Brien MD, Silsbee H, Gronseth G, Lang AE (2021) Dopaminergic therapy for motor symptoms in early Parkinson disease practice guideline summary: a report of the AAN guideline subcommittee. Neurology 97:942–957 - PMC - PubMed
-
- Jenner P, Rocha JF, Ferreira JJ, Rascol O, Soares-da-Silva P (2021) Redefining the strategy for the use of COMT inhibitors in Parkinson’s disease: the role of opicapone. Expert Rev Neurother 21:1019–1033 - PubMed
-
- Stocchi F, Jenner P, Obeso JA (2010) When do levodopa motor fluctuations first appear in Parkinson’s disease? Eur Neurol 63:257–266 - PubMed
-
- Stocchi F, Antonini A, Barone P, Tinazzi M, Zappia M, Onofrj M, Ruggieri S, Morgante L, Bonuccelli U, Lopiano L, Pramstaller P, Albanese A, Attar M, Posocco V, Colombo D, Abbruzzese G, group Ds (2014) Early DEtection of wEaring off in Parkinson disease: the DEEP study. Parkinsonism Relat Disord 20:204–211 - PubMed
-
- Olanow CW, Kieburtz K, Rascol O, Poewe W, Schapira AH, Emre M, Nissinen H, Leinonen M, Stocchi F, Stalevo Reduction in Dyskinesia Evaluation in Parkinson’s Disease I (2013) Factors predictive of the development of Levodopa-induced dyskinesia and wearing-off in Parkinson’s disease. Mov Disord 28:1064–1071 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous