Interleukin-33 promotes intrauterine adhesion formation in mice through the mitogen-activated protein kinase signaling pathway
- PMID: 39164588
- PMCID: PMC11336135
- DOI: 10.1038/s42003-024-06709-1
Interleukin-33 promotes intrauterine adhesion formation in mice through the mitogen-activated protein kinase signaling pathway
Abstract
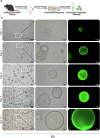
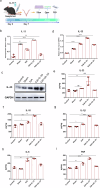
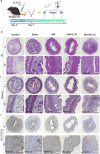
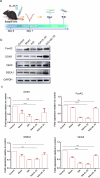
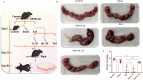
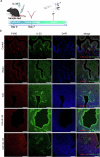
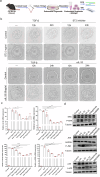
IL-33 belongs to the inflammatory factor family and is closely associated with the inflammatory response. However, its role in the development of intrauterine adhesions (IUAs) remains unclear. In this study, the role of IL-33 in the formation of IUAs after endometrial injury was identified via RNA sequencing after mouse endometrial organoids were transplanted into an IUA mouse model. Major pathological changes in the mouse uterus, consistent with the expression of fibrotic markers, such as TGF-β, were observed in response to treatment with IL-33. This finding may be attributed to activation of the phosphorylation of downstream MAPK signaling pathway components, which are activated by the release of IL-33 in macrophages. Our study provides a novel mechanism for elucidating IUA formation, suggesting a new therapeutic strategy for the prevention and clinical treatment of IUAs.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Hooker, A. B. et al. Systematic review and meta-analysis of intrauterine adhesions after miscarriage: prevalence, risk factors and long-term reproductive outcome. Hum. Reprod.20, 262–278 (2014). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

