The activity-regulated cytoskeleton-associated protein (Arc) functions in a cell type- and sex-specific manner in the adult nucleus accumbens to regulate non-contingent cocaine behaviors
- PMID: 39164860
- PMCID: PMC11335578
- DOI: 10.1111/gbb.12910
The activity-regulated cytoskeleton-associated protein (Arc) functions in a cell type- and sex-specific manner in the adult nucleus accumbens to regulate non-contingent cocaine behaviors
Abstract
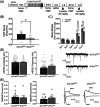
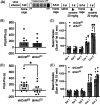
Repeated cocaine use produces adaptations in brain function that contribute to long-lasting behaviors associated with cocaine use disorder (CUD). In rodents, the activity-regulated cytoskeleton-associated protein (Arc) can regulate glutamatergic synaptic transmission, and cocaine regulates Arc expression and subcellular localization in multiple brain regions, including the nucleus accumbens (NAc)-a brain region linked to CUD-related behavior. We show here that repeated, non-contingent cocaine administration in global Arc KO male mice produced a dramatic hypersensitization of cocaine locomotor responses and drug experience-dependent sensitization of conditioned place preference (CPP). In contrast to the global Arc KO mice, viral-mediated reduction of Arc in the adult male, but not female, NAc (shArcNAc) reduced both CPP and cocaine-induced locomotor activity, but without altering basal miniature or evoked glutamatergic synaptic transmission. Interestingly, cell type-specific knockdown of Arc in D1 dopamine receptor-expressing NAc neurons reduced cocaine-induced locomotor sensitization, but not cocaine CPP; whereas, Arc knockdown in D2 dopamine receptor-expressing NAc neurons reduced cocaine CPP, but not cocaine-induced locomotion. Taken together, our findings reveal that global, developmental loss of Arc produces hypersensitized cocaine responses; however, these effects cannot be explained by Arc's function in the adult mouse NAc since Arc is required in a cell type- and sex-specific manner to support cocaine-context associations and locomotor responses.
Keywords: AMPA; Arc/Arg3.1; NMDA; cocaine; conditioned place preference; knockout mouse; medium spiny neurons; nucleus accumbens; sensitization; sex differences.
© 2024 The Author(s). Genes, Brain and Behavior published by International Behavioural and Neural Genetics Society and John Wiley & Sons Ltd.
Conflict of interest statement
All authors report no biomedical financial interests or potential conflicts of interest.
Figures


References
MeSH terms
Substances
Grants and funding
- F32 DA036319/NH/NIH HHS/United States
- R21 MH123883/MH/NIMH NIH HHS/United States
- R01 DA032708/DA/NIDA NIH HHS/United States
- K12 HD055885/NH/NIH HHS/United States
- F32 DA036319/DA/NIDA NIH HHS/United States
- Rappaport Mental Health Research Scholars Program
- P50 DA046373/DA/NIDA NIH HHS/United States
- P20 GM148302/GM/NIGMS NIH HHS/United States
- T32 DA007288/DA/NIDA NIH HHS/United States
- P50 DA046373/NH/NIH HHS/United States
- R21 MH123883/NH/NIH HHS/United States
- T32DA007288/NH/NIH HHS/United States
- R01 DA027664/NH/NIH HHS/United States
- K12 HD055885/HD/NICHD NIH HHS/United States
- R01 DA027664/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials

