Remote monitoring of automated peritoneal dialysis reduces mortality, adverse events and hospitalizations: a cluster-randomized controlled trial
- PMID: 39165115
- PMCID: PMC11997789
- DOI: 10.1093/ndt/gfae188
Remote monitoring of automated peritoneal dialysis reduces mortality, adverse events and hospitalizations: a cluster-randomized controlled trial
Abstract
Background: Remote monitoring (RM) of patients on automated peritoneal dialysis (APD) prevents complications and improves treatment quality. We analyzed the effect of RM-APD on mortality and complications related to cardiovascular disease, fluid overload and insufficient dialysis efficiency.
Methods: In a cluster-randomized, open-label, controlled trial, 21 hospitals with APD programs were assigned to use either RM-APD (10 hospitals; 403 patients) or conventional APD (11 hospitals; 398 patients) for the treatment of adult patients starting PD. Primary outcomes were time to first event of: (i) Composite Index 1 comprising all-cause mortality, first adverse events and hospitalizations of any cause, and (ii) Composite Index 2 comprising cardiovascular mortality, first adverse event and hospitalizations related to cardiovascular disease, fluid overload and insufficient dialysis efficiency. Secondary outcomes were time to first event of individual components of the two composite indices, and rates of adverse events, hospitalizations, unplanned visits and transfer to hemodialysis. Patients were followed for a median of 9.5 months. Primary outcomes were evaluated by competing risk analysis and restricted mean survival time (RMST) analysis.
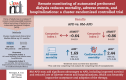
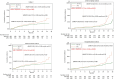
Results: While time to reach Composite Index 1 did not differ between the groups, Composite Index 2 was reached earlier (ΔRMST: -0.86 months; P = .02), and all-cause mortality [55 vs 33 deaths, P = .01; sub-hazard ratio (sHR) 1.69 (95% confidence interval 1.39-2.05), P < .001] and hospitalizations of any cause were higher in APD group than in RM-APD as were cardiovascular deaths [24 vs 13 deaths, P = .05; sHR 2.44 (95% confidence interval 1.72-3.45), P < .001] and rates of adverse events and hospitalizations related to cardiovascular disease, fluid overload or insufficient dialysis efficiency. Dropouts were more common in the APD group (131 vs 110, P = .048).
Conclusions: This randomized controlled trial shows that RM may add significant advantages to APD, including improved survival and reduced rate of adverse events and hospitalizations, which can favorably impact the acceptance and adoption of the therapy.
Keywords: adverse events; automated peritoneal dialysis; cluster randomized control trial; mortality; remote patient monitoring.
© The Author(s) 2024. Published by Oxford University Press on behalf of the ERA.
Conflict of interest statement
R.P. has received support from Baxter Healthcare Corporation for clinical research, conferences and advisory board meetings. B.L. has been employed by Baxter Healthcare Corporation. Baxter Novum is the result of a grant of Baxter Healthcare Corporation to Karolinska Institutet. Baxter Healthcare Corporation did not have any role in study design, data collection or data analysis.
Figures

References
-
- Popovich RP, Moncrief JW, Nolph KDet al. . Continuous ambulatory peritoneal dialysis. Ann Intern Med 1978;88:449–56. - PubMed
-
- Mendez-Duran A. Evolution of renal replacement therapy in Mexico in the last 10 years. Nefrologia (Engl Ed) 2021;41:82–3. - PubMed
-
- United States Renal Data System . USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. 2020. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD.
-
- Jiwakanon S, Chiu YW, Kalantar-Zadeh Ket al. . Peritoneal dialysis: an underutilized modality. Curr Opin Nephrol Hypertens 2010;19:573–7. - PubMed
-
- Mehrotra R. Choice of dialysis modality. Kidney Int 2011;80:909–11. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

