Absorbable metal stents for vascular use in pediatric cardiology: progress and outlook
- PMID: 39165257
- PMCID: PMC11334478
- DOI: 10.3389/fcvm.2024.1410305
Absorbable metal stents for vascular use in pediatric cardiology: progress and outlook
Abstract
The past five years have yielded impressive advancements in fully absorbable metal stent technology. The desired ultimate ability for such devices to treat a vascular stenosis without long-term device-related complications or impeding future treatment continues to evoke excitement in clinicians and engineers alike. Nowhere is the need for fully absorbable metal stents greater than in patients experiencing vascular anomalies associated with congenital heart disease (CHD). Perhaps not surprisingly, commercially available absorbable metal stents have been implanted in pediatric cardiology patients with conditions ranging from pulmonary artery and vein stenosis to coarctation of the aorta and conduit/shunt reconstructions. Despite frequent short term procedural success, device performance has missed the mark with the commercially available devices not achieving degradation benchmarks for given applications. In this review we first provide a general overview detailing the theory of absorbable metal stents, and then review recent clinical use in CHD patients since the release of current-generation absorbable metal stents around 2019. We also discuss the challenges and our center's experience associated with the use of absorbable metal stents in this pediatric population. Lastly, we present potential directions for future engineering endeavors to mitigate existing challenges.
Keywords: absorbable metals; iron; magnesium; pediatric stents; zinc.
© 2024 McLennan, Maldonado, Foerster, Handler, LaDisa, Gudausky and Guillory II.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


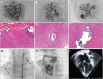
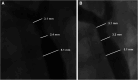
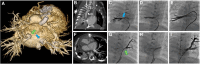
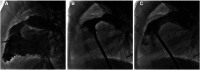
References
-
- Tamai H, Igaki K, Tsuji T, Kyo E, Kosuga K, Kawashima A, et al. A biodegradable poly-l-lactic acid coronary stent in the porcine coronary artery. J Interv Cardiol. (1999) 12(6):443–50. 10.1111/j.1540-8183.1999.tb00673.x - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

