Efficient nitrite determination by electrochemical approach in liquid phase with ultrasonically prepared gold-nanoparticle-conjugated conducting polymer nanocomposites
- PMID: 39165336
- PMCID: PMC11333211
- DOI: 10.3389/fchem.2024.1358353
Efficient nitrite determination by electrochemical approach in liquid phase with ultrasonically prepared gold-nanoparticle-conjugated conducting polymer nanocomposites
Abstract
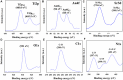
An electrochemical nitrite sensor probe is introduced herein using a modified flat glassy carbon electrode (GCE) and SrTiO3 material doped with spherical-shaped gold nanoparticles (Au-NPs) and polypyrrole carbon (PPyC) at a pH of 7.0 in a phosphate buffer solution. The nanocomposites (NCs) containing Au-NPs, PPyC, and SrTiO3 were synthesized by ultrasonication, and their properties were thoroughly characterized through structural, elemental, optical, and morphological analyses with various conventional spectroscopic methods, such as field-emission scanning electron microscopy, energy-dispersive X-ray spectroscopy, high-resolution transmission electron microscopy, powder X-ray diffraction, X-ray photoelectron spectroscopy, and Brunauer-Emmett-Teller method. The peak currents due to nitrite oxidation were characterized in detail and analyzed using conventional cyclic voltammetry (CV) as well as differential pulse voltammetry (DPV) under ambient conditions. The sensor response increased significantly from 0.15 to 1.5 mM of nitrite ions, and the sensor was fabricated by coating a conducting agent (PEDOT:PSS) on the GCE to obtain the Au-NPs/PPyC/SrTiO3 NCs/PEDOT:PSS/GCE probe. The sensor's sensitivity was determined as 0.5 μA/μM∙cm2 from the ratio of the slope of the linear detection range by considering the active surface area (0.0316 cm2) of the flat GCE. In addition, the limit of detection was determined as 20.00 ± 1.00 µM, which was found to be satisfactory. The sensor's stability, pH optimization, and reliability were also evaluated in these analyses. Overall, the sensor results were found to be satisfactory. Real environmental samples were then analyzed to evaluate the sensor's reliability through DPV, and the results showed that the proposed novel electrochemical sensor holds great promise for mitigating water contamination in the real samples with the lab-made Au-NPs/PPyC/SrTiO3 NC. Thus, this study provides valuable insights for improving sensors for broad environmental monitoring applications using the electrochemical approach.
Keywords: Au-NPs/PPyC/SrTiO3 nanocomposites; differential pulse voltammetry; environmental remediation; glassy carbon electrode; nitrite detection.
Copyright © 2024 Faisal, Alam, Ahmed, Asiri, Algethami, Altholami, Harraz and Rahman.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures










References
-
- Amini N., Maleki A., Maleki P. (2021). Electrochemical detection of nitrate ions via reduction of NO2− and oxidation of NO reactions based on Cu@TiO2 coreshell/nafion/polyalizarin immobilized electrode. Mater. Chem. Phys. 264, 124384. 10.1016/j.matchemphys.2021.124384 - DOI
-
- Asiri A. M., Adeosun W. A., Rahman M. M. (2020). Development of highly efficient non-enzymatic nitrite sensor using La2CuO4 nanoparticles. Microchem. J. 159, 105527. 10.1016/j.microc.2020.105527 - DOI
-
- Bahadoran Z., Mirmiran P., Jeddi S., Azizi F., Ghasemi A., Hadaegh F. (2016). Nitrate and nitrite content of vegetables, fruits, grains, legumes, dairy products, meats and processed meats. J. Food Compos. Anal. 51, 93–105. 10.1016/j.jfca.2016.06.006 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

