Differences in Interfacial Reactivity of Graphite and Lithium Metal Battery Electrodes Investigated Via Operando Gas Analysis
- PMID: 39165483
- PMCID: PMC11331504
- DOI: 10.1021/acs.jpcc.4c03656
Differences in Interfacial Reactivity of Graphite and Lithium Metal Battery Electrodes Investigated Via Operando Gas Analysis
Abstract
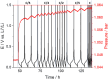
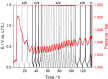
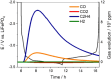
Gases evolved from lithium batteries can drastically affect their performance and safety; for example, cell swelling is a serious safety issue. Here, we combine operando pressure measurements and online electrochemical mass spectrometry measurements to identify the nature and quantity of gases formed in batteries with graphite and lithium metal electrodes. We demonstrate that ethylene, a main gas evolved in SEI formation reactions, is quickly consumed at lithium metal electrodes unless they have been pretreated in the electrolyte. Polyolefins such as polyethylene are suggested as the possible reaction product from ethylene consumption, evidencing another pathway of SEI formation that had been previously overlooked because it does not produce any gas product.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Balakrishnan P. G.; Ramesh R.; Prem Kumar T. Safety Mechanisms in Lithium-Ion Batteries. J. Power Sources 2006, 155 (2), 401–414. 10.1016/j.jpowsour.2005.12.002. - DOI
-
- Wang Q.; Ping P.; Zhao X.; Chu G.; Sun J.; Chen C. Thermal Runaway Caused Fire and Explosion of Lithium Ion Battery. J. Power Sources 2012, 208, 210–224. 10.1016/j.jpowsour.2012.02.038. - DOI
-
- Choi D.; Shamim N.; Crawford A.; Huang Q.; Vartanian C. K.; Viswanathan V. V.; Paiss M. D.; Alam M. J. E.; Reed D. M.; Sprenkle V. L. Li-Ion Battery Technology for Grid Application. J. Power Sources 2021, 511, 230419.10.1016/j.jpowsour.2021.230419. - DOI
-
- Kong W.; Li H.; Huang X.; Chen L. Gas Evolution Behaviors for Several Cathode Materials in Lithium-Ion Batteries. J. Power Sources 2005, 142 (1–2), 285–291. 10.1016/j.jpowsour.2004.10.008. - DOI
-
- Aiken C. P.; Xia J.; Wang D. Y.; Stevens D. A.; Trussler S.; Dahn J. R. An Apparatus for the Study of In Situ Gas Evolution in Li-Ion Pouch Cells. J. Electrochem. Soc. 2014, 161 (10), A1548–A1554. 10.1149/2.0151410jes. - DOI
LinkOut - more resources
Full Text Sources
