Botulinum toxin type A-targeted SPP1 contributes to neuropathic pain by the activation of microglia pyroptosis
- PMID: 39165552
- PMCID: PMC11331382
- DOI: 10.5498/wjp.v14.i8.1254
Botulinum toxin type A-targeted SPP1 contributes to neuropathic pain by the activation of microglia pyroptosis
Abstract
Background: Neuropathic pain (NP) is the primary symptom of various neurological conditions. Patients with NP often experience mood disorders, particularly depression and anxiety, that can severely affect their normal lives. Microglial cells are associated with NP. Excessive inflammatory responses, especially the secretion of large amounts of pro-inflammatory cytokines, ultimately lead to neuroinflammation. Microglial pyroptosis is a newly discovered form of inflammatory cell death associated with immune responses and inflammation-related diseases of the central nervous system.
Aim: To investigate the effects of botulinum toxin type A (BTX-A) on microglial pyroptosis in terms of NP and associated mechanisms.
Methods: Two models, an in vitro lipopolysaccharide (LPS)-stimulated microglial cell model and a selective nerve injury model using BTX-A and SPP1 knockdown treatments, were used. Key proteins in the pyroptosis signaling pathway, NLRP3-GSDMD, were assessed using western blotting, real-time quantitative polymerase chain reaction, and immunofluorescence. Inflammatory factors [interleukin (IL)-6, IL-1β, and tumor necrosis factor (TNF)-α] were assessed using enzyme-linked immunosorbent assay. We also evaluated microglial cell proliferation and apoptosis. Furthermore, we measured pain sensation by assessing the delayed hind paw withdrawal latency using thermal stimulation.
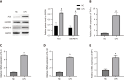
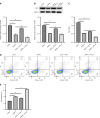
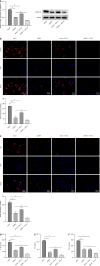
Results: The expression levels of ACS and GSDMD-N and the mRNA expression of TNF-α, IL-6, and IL-1β were enhanced in LPS-treated microglia. Furthermore, SPP1 expression was also induced in LPS-treated microglia. Notably, BTX-A inhibited SPP1 mRNA and protein expression in the LPS-treated microglia. Additionally, depletion of SPP1 or BTX-A inhibited cell viability and induced apoptosis in LPS-treated microglia, whereas co-treatment with BTX-A enhanced the effect of SPP1 short hairpin (sh)RNA in LPS-treated microglia. Finally, SPP1 depletion or BTX-A treatment reduced the levels of GSDMD-N, NLPRP3, and ASC and suppressed the production of inflammatory factors.
Conclusion: Notably, BTX-A therapy and SPP1 shRNA enhance microglial proliferation and apoptosis and inhibit microglial death. It improves pain perception and inhibits microglial activation in rats with selective nerve pain.
Keywords: Botulinum toxin A; Microglia; Neuropathic pain; Pyroptosis; SPP1.
©The Author(s) 2023. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
Figures

References
-
- van Hecke O, Austin SK, Khan RA, Smith BH, Torrance N. Neuropathic pain in the general population: a systematic review of epidemiological studies. Pain. 2014;155:654–662. - PubMed
-
- Argoff CE. The coexistence of neuropathic pain, sleep, and psychiatric disorders: a novel treatment approach. Clin J Pain. 2007;23:15–22. - PubMed
-
- Magrinelli F, Zanette G, Tamburin S. Neuropathic pain: diagnosis and treatment. Pract Neurol. 2013;13:292–307. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

