Iodinated gadolinium-gold nanomaterial as a multimodal contrast agent for cartilage tissue imaging
- PMID: 39165611
- PMCID: PMC11335359
- DOI: 10.1063/5.0215273
Iodinated gadolinium-gold nanomaterial as a multimodal contrast agent for cartilage tissue imaging
Abstract
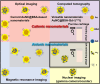
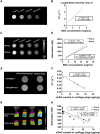
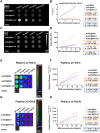
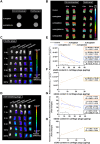
Cartilage damage, a common cause of osteoarthritis, requires medical imaging for accurate diagnosis of pathological changes. However, current instruments can acquire limited imaging information due to sensitivity and resolution issues. Therefore, multimodal imaging is considered an alternative strategy to provide valuable images and analyzes from different perspectives. Among all biomaterials, gold nanomaterials not only exhibit outstanding benefits as drug carriers, in vitro diagnostics, and radiosensitizers, but are also widely used as contrast agents, particularly for tumors. However, their potential for imaging cartilage damage is rarely discussed. In this study, we developed a versatile iodinated gadolinium-gold nanomaterial, AuNC@BSA-Gd-I, and its radiolabeled derivative, AuNC@BSA-Gd-131I, for cartilage detection. With its small size, negative charge, and multimodal capacities, the probe can penetrate damaged cartilage and be detected or visualized by computed tomography, MRI, IVIS, and gamma counter. Additionally, the multimodal imaging potential of AuNC@BSA-Gd-I was compared to current multifunctional gold nanomaterials containing similar components, including anionic AuNC@BSA, AuNC@BSA-I, and AuNC@BSA-Gd as well as cationic AuNC@CBSA. Due to their high atomic numbers and fluorescent emission, AuNC@BSA nanomaterials could provide fundamental multifunctionality for imaging. By further modifying AuNC@BSA with additional imaging materials, their application could be extended to various types of medical imaging instruments. Nonetheless, our findings showed that each of the current nanomaterials exhibited excellent abilities for imaging cartilage with their predominant imaging modalities, but their versatility was not comparable to that of AuNC@BSA-Gd-I. Thus, AuNC@BSA-Gd-I could be served as a valuable tool in multimodal imaging strategies for cartilage assessment.
© 2024 Author(s).
Conflict of interest statement
The authors have no conflicts to disclose.
Figures



Similar articles
-
Gold nanomaterials capped with bovine serum albumin for cell and extracellular vesicle imaging.Nanotechnology. 2025 Jan 9;36(10). doi: 10.1088/1361-6528/ada3da. Nanotechnology. 2025. PMID: 39780321
-
A Gold Nanocage Probe Targeting Survivin for the Diagnosis of Pancreatic Cancer.Pharmaceutics. 2023 May 19;15(5):1547. doi: 10.3390/pharmaceutics15051547. Pharmaceutics. 2023. PMID: 37242788 Free PMC article.
-
BSA-Bioinspired Gadolinium Hybrid-Functionalized Hollow Gold Nanoshells for NIRF/PA/CT/MR Quadmodal Diagnostic Imaging-Guided Photothermal/Photodynamic Cancer Therapy.ACS Appl Mater Interfaces. 2017 Nov 22;9(46):40017-40030. doi: 10.1021/acsami.7b11926. Epub 2017 Nov 8. ACS Appl Mater Interfaces. 2017. PMID: 29087183
-
Imaging of knee osteoarthritis: a review of multimodal diagnostic approach.Quant Imaging Med Surg. 2023 Nov 1;13(11):7582-7595. doi: 10.21037/qims-22-1392. Epub 2023 Apr 7. Quant Imaging Med Surg. 2023. PMID: 37969633 Free PMC article. Review.
-
Gold nanocluster-based fluorescent sensors for in vitro and in vivo ratiometric imaging of biomolecules.Phys Chem Chem Phys. 2023 Aug 23;25(33):21787-21801. doi: 10.1039/d3cp02714g. Phys Chem Chem Phys. 2023. PMID: 37577965 Review.
Cited by
-
Visual analysis of emerging topics and trends in contrast agent extravasation research in medical imaging: a bibliometric study using CiteSpace and VOSviewer.Front Med (Lausanne). 2025 Feb 19;12:1472637. doi: 10.3389/fmed.2025.1472637. eCollection 2025. Front Med (Lausanne). 2025. PMID: 40046933 Free PMC article.
References
-
- Casale J. and Crane J. S., in StatPearls (StatPearls Publishing, Treasure Island, FL, 2021), https://pubmed.ncbi.nlm.nih.gov/31335015/.
LinkOut - more resources
Full Text Sources

