Targeting the Cascade Amplification of Macrophage Colony-stimulating Factor to Alleviate the Immunosuppressive Effects Following Radiotherapy
- PMID: 39165639
- PMCID: PMC11334716
- DOI: 10.34133/research.0450
Targeting the Cascade Amplification of Macrophage Colony-stimulating Factor to Alleviate the Immunosuppressive Effects Following Radiotherapy
Abstract
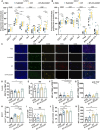
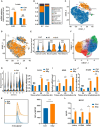
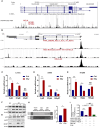
Radiotherapy (RT) serves as the primary treatment for solid tumors. Its potential to incite an immune response against tumors both locally and distally profoundly impacts clinical outcomes. However, RT may also promote the accumulation of immunosuppressive cytokines and immunosuppressive cells, greatly impeding the activation of antitumor immune responses and substantially limiting the effectiveness of RT. Therefore, regulating post-RT immunosuppression to steer the immune milieu toward heightened activation potentially enhances RT's therapeutic potential. Cytokines, potent orchestrators of diverse cellular responses, play a pivotal role in regulating this immunosuppressive response. Identifying and promptly neutralizing early released immunosuppressive cytokines are a crucial development in augmenting RT's immunomodulatory effects. To this end, we conducted a screen of immunosuppressive cytokines following RT and identified macrophage colony-stimulating factor (MCSF) as an early up-regulated and persistent immune suppressor. Single-cell sequencing revealed that the main source of up-regulated MCSF derived from tumor cells. Mechanistic exploration revealed that irradiation-dependent phosphorylation of the p65 protein facilitated its binding to the MCSF gene promoter, enhancing transcription. Knockdown and chemical inhibitor experiments conclusively demonstrated that suppressing tumor cell-derived MCSF amplifies RT's immune-activating effects, with optimal results achieved by early MCSF blockade after irradiation. Additionally, we validated that MCSF acted on macrophages, inducing the secretion of a large number of inhibitory cytokines. In summary, we propose a novel approach to enhance the immune activation effects of RT by blocking the MCSF-CSF1R signaling pathway early after irradiation.
Copyright © 2024 Zhiyun Liao et al.
Conflict of interest statement
Competing interests: The authors declare that they have no competing interests.
Figures




References
-
- Herrera FG, Bourhis J, Coukos G. Radiotherapy combination opportunities leveraging immunity for the next oncology practice. CA Cancer J Clin. 2017;67(1):65–85. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

