Targeting lysosomes by design: novel N-acridine thiosemicarbazones that enable direct detection of intracellular drug localization and overcome P-glycoprotein (Pgp)-mediated resistance
- PMID: 39165729
- PMCID: PMC11331336
- DOI: 10.1039/d4sc04339a
Targeting lysosomes by design: novel N-acridine thiosemicarbazones that enable direct detection of intracellular drug localization and overcome P-glycoprotein (Pgp)-mediated resistance
Abstract
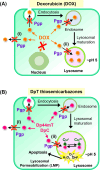
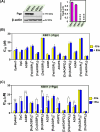
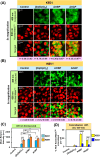
Innovative N-acridine thiosemicarbazones (NATs) were designed along with their iron(iii), copper(ii), and zinc(ii) complexes. Lysosomal targeting was promoted by specifically incorporating the lysosomotropic Pgp substrate, acridine, into the thiosemicarbazone scaffold to maintain the tridentate N, N, S-donor system. The acridine moiety enables a significant advance in thiosemicarbazone design, since: (1) it enables tracking of the drugs by confocal microscopy using its inherent fluorescence; (2) it is lysosomotropic enabling lysosomal targeting; and (3) as acridine is a P-glycoprotein (Pgp) substrate, it facilitates lysosomal targeting, resulting in the drug overcoming Pgp-mediated resistance. These new N-acridine analogues are novel, and this is the first time that acridine has been specifically added to the thiosemicarbazone framework to achieve the three important properties above. These new agents displayed markedly greater anti-proliferative activity against resistant Pgp-expressing cells than very low Pgp-expressing cells. The anti-proliferative activity of NATs against multiple Pgp-positive cancer cell-types (colon, lung, and cervical carcinoma) was abrogated by the third generation Pgp inhibitor, Elacridar, and also Pgp siRNA that down-regulated Pgp. Confocal microscopy demonstrated that low Pgp in KB31 (-Pgp) cells resulted in acridine's proclivity for DNA intercalation promoting NAT nuclear-targeting. In contrast, high Pgp in KBV1 (+Pgp) cells led to NAT lysosomal sequestration, preventing its nuclear localisation. High Pgp expression in KBV1 (+Pgp) cells resulted in co-localization of NATs with the lysosomal marker, LysoTracker™, that was significantly (p < 0.001) greater than the positive control, the di-2-pyridylketone-4-cyclohexyl-4-methyl-3-thiosemicarbazone (DpC) Zn(ii) complex, [Zn(DpC)2]. Incorporation of acridine into the thiosemicarbazone scaffold led to Pgp-mediated transport into lysosomes to overcome Pgp-resistance.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no competing conflict of interest.
Figures



Similar articles
-
A mechanism for overcoming P-glycoprotein-mediated drug resistance: novel combination therapy that releases stored doxorubicin from lysosomes via lysosomal permeabilization using Dp44mT or DpC.Cell Death Dis. 2016 Dec 1;7(12):e2510. doi: 10.1038/cddis.2016.381. Cell Death Dis. 2016. PMID: 27906178 Free PMC article.
-
Di-2-pyridylketone 4,4-dimethyl-3-thiosemicarbazone (Dp44mT) overcomes multidrug resistance by a novel mechanism involving the hijacking of lysosomal P-glycoprotein (Pgp).J Biol Chem. 2015 Apr 10;290(15):9588-603. doi: 10.1074/jbc.M114.631283. Epub 2015 Feb 26. J Biol Chem. 2015. PMID: 25720491 Free PMC article.
-
Glucose Modulation Induces Lysosome Formation and Increases Lysosomotropic Drug Sequestration via the P-Glycoprotein Drug Transporter.J Biol Chem. 2016 Feb 19;291(8):3796-820. doi: 10.1074/jbc.M115.682450. Epub 2015 Nov 24. J Biol Chem. 2016. PMID: 26601947 Free PMC article.
-
Turning the gun on cancer: Utilizing lysosomal P-glycoprotein as a new strategy to overcome multi-drug resistance.Free Radic Biol Med. 2016 Jul;96:432-45. doi: 10.1016/j.freeradbiomed.2016.04.201. Epub 2016 May 3. Free Radic Biol Med. 2016. PMID: 27154979 Review.
-
Anticancer Thiosemicarbazones: Chemical Properties, Interaction with Iron Metabolism, and Resistance Development.Antioxid Redox Signal. 2019 Mar 10;30(8):1062-1082. doi: 10.1089/ars.2017.7487. Epub 2018 Feb 26. Antioxid Redox Signal. 2019. PMID: 29334758 Review.
Cited by
-
Lysosomes and LAMPs as Autophagy Drivers of Drug Resistance in Colorectal Cancer.Cells. 2025 Apr 11;14(8):574. doi: 10.3390/cells14080574. Cells. 2025. PMID: 40277899 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

