Elucidating the effects of nitrogen and phosphorus co-doped carbon on complex spinel NiFe2O4 towards oxygen reduction reaction in alkaline media
- PMID: 39166028
- PMCID: PMC11334865
- DOI: 10.1016/j.heliyon.2024.e35483
Elucidating the effects of nitrogen and phosphorus co-doped carbon on complex spinel NiFe2O4 towards oxygen reduction reaction in alkaline media
Abstract
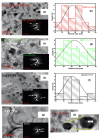
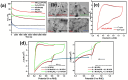
The study presents for the first time complex spinel NiFe2O4 nanoparticles supported on nitrogen and phosphorus co-doped carbon nanosheets (NPCNS) prepared using sol gel and the carbonization of graphitic carbon nitride with lecithin as a highly active and durable electrocatalyst for oxygen reduction reaction. The physicochemical properties of complex spinel NiFe2O4 on NPCNS and subsequent nanomaterials were investigated using techniques such as X-ray diffraction, Fourier transform infrared spectroscopy, X-ray photoelectron spectroscopy, and transmission electron microscopy. The electrochemical activity of the electrocatalysts was evaluated using hydrodynamic linear sweep voltammetry, cyclic voltammetry, electrochemical impedance spectroscopy, and chronoamperometry. The electrocatalytic performance of the NiFe2O4/NPCNS nanohybrid electrocatalyst is dominated by the 4e- transfer mechanism, with an onset potential of 0.92 V vs. RHE, which is closer to that of the Pt/C, and a current density of 7.81 mA/cm2 that far exceeds that of the Pt/C. The nanohybrid demonstrated the best stability after 14 400 s, outstanding durability after 521 cycles, and the best ability to oxidize methanol and remove CO from its active sites during CO tolerance studies. This improved catalytic activity can be attributed to small nanoparticle sizes of the unique complex spinel nickel ferrite structure, N-Fe/Ni coordination of nanocomposite, high dispersion, substantial ECSA of 47.03 mF/cm2, and synergy caused by strong metal-support and electronic coupling interactions.
© 2024 Published by Elsevier Ltd.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






References
-
- Ren S., Ma S., Yang Y., Mao Q., Hao C. Hydrothermal synthesis of Fe2O3/polypyrrole/graphene oxide composites as highly efficient electrocatalysts for oxygen reduction reaction in alkaline electrolyte, 178. Electrochim. Acta. 2015:179–189. doi: 10.1016/j.electacta.2015.07.181. - DOI
-
- Xian F., Gao L., Zhang Z., Zhang H., Dong S., Cui G. N, P dual-doped multi-wrinkled nanosheets prepared from the egg crude lecithin as the efficient metal-free electrocatalyst for oxygen reduction reaction. Appl. Surf. Sci. 2019;476:76–83. doi: 10.1016/j.apsusc.2018.12.293. - DOI
LinkOut - more resources
Full Text Sources

