Formation of S-Bearing Complex Organic Molecules in Interstellar Clouds via Ice Reactions with C2H2, HS, and Atomic H
- PMID: 39166258
- PMCID: PMC11331529
- DOI: 10.1021/acsearthspacechem.4c00150
Formation of S-Bearing Complex Organic Molecules in Interstellar Clouds via Ice Reactions with C2H2, HS, and Atomic H
Abstract
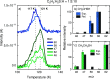
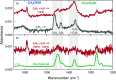
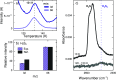
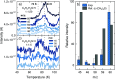
The chemical network governing interstellar sulfur has been the topic of unrelenting discussion for the past few decades due to the conspicuous discrepancy between its expected and observed abundances in different interstellar environments. More recently, the astronomical detections of CH3CH2SH and CH2CS highlighted the importance of interstellar formation routes for sulfur-bearing organic molecules with two carbon atoms. In this work, we perform a laboratory investigation of the solid-state chemistry resulting from the interaction between C2H2 molecules and SH radicals-both thought to be present in interstellar icy mantles-at 10 K. Reflection absorption infrared spectroscopy and quadrupole mass spectrometry combined with temperature-programmed desorption experiments are employed as analytical techniques. We confirm that SH radicals can kick-start a sulfur reaction network under interstellar cloud conditions and identify at least six sulfurated products: CH3CH2SH, CH2CHSH, HSCH2CH2SH, H2S2, and tentatively CH3CHS and CH2CS. Complementarily, we utilize computational calculations to pinpoint the reaction routes that play a role in the chemical network behind our experimental results. The main sulfur-bearing organic molecule formed under our experimental conditions is CH3CH2SH, and its formation yield increases with the ratios of H to other reactants. It serves as a sink to the sulfur budget within the network, being formed at the expense of the other unsaturated products. The astrophysical implications of the chemical network proposed here are discussed.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Müller H. S. P.; Schlöder F.; Stutzki J.; Winnewisser G. The Cologne Database for Molecular Spectroscopy, CDMS: a useful tool for astronomers and spectroscopists. J. Mol. Struct. 2005, 742, 215–227. 10.1016/j.molstruc.2005.01.027. - DOI
-
- McGuire B. A. 2021 census of interstellar, circumstellar, extragalactic, protoplanetary disk, and exoplanetary molecules. Astrophysical Journal Supplement Series 2022, 259, 30.10.3847/1538-4365/ac2a48. - DOI
-
- Herbst E.; van Dishoeck E. F. Complex Organic Interstellar Molecules. Annual Review of Astronomy and Astrophysics 2009, 47, 427–480. 10.1146/annurev-astro-082708-101654. - DOI
-
- Öberg K. I.; Bottinelli S.; Jørgensen J. K.; van Dishoeck E. F. A Cold Complex Chemistry Toward the Low-mass Protostar B1-b: Evidence for Complex Molecule Production in Ices. Astrophysical Journal 2010, 716, 825–834. 10.1088/0004-637X/716/1/825. - DOI
-
- Bacmann A.; Taquet V.; Faure A.; Kahane C.; Ceccarelli C. Detection of complex organic molecules in a prestellar core: a new challenge for astrochemical models. Astron. Astrophys. 2012, 541, L12.10.1051/0004-6361/201219207. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous
