Role of Source, Mineralogy, and Organic Complexation on Lability and Fe Isotopic Composition of Terrestrial Fe sources to the Gulf of Alaska
- PMID: 39166260
- PMCID: PMC11331515
- DOI: 10.1021/acsearthspacechem.3c00338
Role of Source, Mineralogy, and Organic Complexation on Lability and Fe Isotopic Composition of Terrestrial Fe sources to the Gulf of Alaska
Abstract
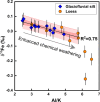
Iron (Fe) is a key trace nutrient supporting marine primary production, and its deposition in the surface ocean can impact multiple biogeochemical cycles. Understanding Fe cycling in the subarctic is key for tracking the fate of particulate-bound sources of oceans in a changing climate. Recently, Fe isotope ratios have been proposed as a potential tool to trace sources of Fe to the marine environment. Here, we investigate the Fe isotopic composition of terrestrial sources of Fe including glacial sediment, loess, volcanic ash, and wildfire aerosols, all from Alaska. Results show that the δ56Fe values of glaciofluvial silt, glacial dissolved load, volcanic ash, and wildfire aerosols fall in a restricted range of δ56Fe values from -0.02 to +0.12‰, in contrast to the broader range of Fe isotopic compositions observed in loess, -0.50 to +0.13‰. The Fe isotopic composition of the dissolved load of glacial meltwater was consistently lighter compared to its particulate counterpart. The 'aging' (exposure to environmental conditions) of volcanic ash did not significantly fractionate the Fe isotopic composition. The Fe isotopic composition of wildfire aerosols collected during an active fire season in Alaska in the summer of 2019 was not significantly fractionated from those of the average upper continental crust composition. We find that the δ56Fe values of loess (<5 μm fraction) were more negative (-0.32 to +0.05‰) with respect to all samples measured here, had the highest proportion of easily reducible Fe (5.9-59.6%), and were correlated with the degree of chemical weathering and organic matter content. Transmission electron spectroscopy measurements indicate an accumulation of amorphous Fe phases in the loess. Our results indicate that Fe isotopes can be related to Fe lability when in the presence of organic matter and that higher organic matter content is associated with a distinctly more negative Fe isotope signature likely due to Fe-organic complexation.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures







References
-
- Martin J. H.; Fitzwater S. E. Iron deficiency limits phytoplankton growth in the north-east Pacific subarctic. Nature 1988, 331 (6154), 341–343. 10.1038/331341a0. - DOI
-
- Charlson R. J.; Lovelock J. E.; Andreae M. O.; Warren S. G. Oceanic phytoplankton, atmospheric sulphur, cloud albedo and climate. Nature 1987, 326 (6114), 655–661. 10.1038/326655a0. - DOI
-
- Coale K. H.; Johnson K. S.; Chavez F. P.; Buesseler K. O.; Barber R. T.; Brzezinski M. A.; Cochlan W. P.; Millero F. J.; Falkowski P. G.; Bauer J. E. Southern Ocean iron enrichment experiment: carbon cycling in high-and low-Si waters. Science 2004, 304 (5669), 408–414. 10.1126/science.1089778. - DOI - PubMed
-
- Tagliabue A.; Aumont O.; Bopp L. The impact of different external sources of iron on the global carbon cycle. Geophys. Res. Lett. 2014, 41 (3), 920–926. 10.1002/2013GL059059. - DOI
-
- Joos F.; Sarmiento J. L.; Siegenthaler U. Estimates of the effect of Southern Ocean iron fertilization on atmospheric CO 2 concentrations. Nature 1991, 349 (6312), 772–775. 10.1038/349772a0. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
