Roseburia intestinalis-derived extracellular vesicles ameliorate colitis by modulating intestinal barrier, microbiome, and inflammatory responses
- PMID: 39166405
- PMCID: PMC11336657
- DOI: 10.1002/jev2.12487
Roseburia intestinalis-derived extracellular vesicles ameliorate colitis by modulating intestinal barrier, microbiome, and inflammatory responses
Abstract
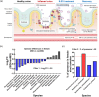
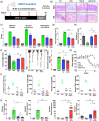
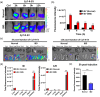
Inflammatory bowel disease (IBD) is a chronic disorder characterized by recurrent gastrointestinal inflammation, lacking a precise aetiology and definitive cure. The gut microbiome is vital in preventing and treating IBD due to its various physiological functions. In the interplay between the gut microbiome and human health, extracellular vesicles secreted by gut bacteria (BEVs) are key mediators. Herein, we explore the role of Roseburia intestinalis (R)-derived EVs (R-EVs) as potent anti-inflammatory mediators in treating dextran sulfate sodium-induced colitis. R was selected as an optimal BEV producer for IBD treatment through ANCOM analysis. R-EVs with a 76 nm diameter were isolated from R using a tangential flow filtration system. Orally administered R-EVs effectively accumulated in inflamed colonic tissues and increased the abundance of Bifidobacterium on microbial changes, inhibiting colonic inflammation and prompting intestinal recovery. Due to the presence of Ile-Pro-Ile in the vesicular structure, R-EVs reduced the DPP4 activity in inflamed colonic tissue and increased the active GLP-1, thereby downregulating the NFκB and STAT3 via the PI3K pathway. Our results shed light on the impact of BEVs on intestinal recovery and gut microbiome alteration in treating IBD.
Keywords: Ile‐Pro‐Ile; R. intestinalis; bacterial extracellular vesicle; inflammatory bowel disease; microbiome.
© 2024 The Author(s). Journal of Extracellular Vesicles published by Wiley Periodicals LLC on behalf of International Society for Extracellular Vesicles.
Conflict of interest statement
K.Y.C is the founder of NVience Inc. S.Y.C, and J.S.P are employees of NVience Inc. The other authors declare no competing interests.
Figures



References
-
- Behl, T. , Sehgal, A. , Grover, M. , Singh, S. , Sharma, N. , Bhatia, S. , Al‐Harrasi, A. , Aleya, L. , & Bungau, S. (2021). Uncurtaining the pivotal role of ABC transporters in diabetes mellitus. Environmental science and pollution research international, 28(31), 41533–41551. 10.1007/s11356-021-14675-y - DOI - PubMed
-
- Belenguer, A. , Duncan, S. H. , Calder, A. G. , Holtrop, G. , Louis, P. , Lobley, G. E. , & Flint, H. J. (2006). Two routes of metabolic cross‐feeding between Bifidobacterium adolescentis and butyrate‐producing anaerobes from the human gut. Applied and environmental microbiology, 72(5), 3593–3599. 10.1128/AEM.72.5.3593-3599.2006 - DOI - PMC - PubMed
-
- Beloqui, A. , Coco, R. , Alhouayek, M. , Solinís, M. Á. , Rodríguez‐Gascón, A. , Muccioli, G. G. , & Préat, V. (2013). Budesonide‐loaded nanostructured lipid carriers reduce inflammation in murine DSS‐induced colitis. International journal of pharmaceutics, 454(2), 775–783. 10.1016/j.ijpharm.2013.05.017 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

