Aloperine Suppresses Cancer Progression by Interacting with VPS4A to Inhibit Autophagosome-lysosome Fusion in NSCLC
- PMID: 39166458
- PMCID: PMC11336898
- DOI: 10.1002/advs.202308307
Aloperine Suppresses Cancer Progression by Interacting with VPS4A to Inhibit Autophagosome-lysosome Fusion in NSCLC
Abstract
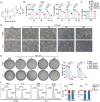
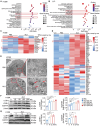
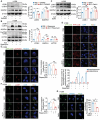
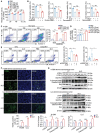
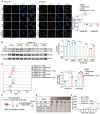
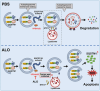
Aloperine (ALO), a quinolizidine-type alkaloid isolated from a natural Chinese herb, has shown promising antitumor effects. Nevertheless, its common mechanism of action and specific target remain elusive. Here, it is demonstrated that ALO inhibits the proliferation and migration of non-small cell lung cancer cell lines in vitro and the tumor development in several mouse tumor models in vivo. Mechanistically, ALO inhibits the fusion of autophagosomes with lysosomes and the autophagic flux, leading to the accumulation of sequestosome-1 (SQSTM1) and production of reactive oxygen species (ROS), thereby inducing tumor cell apoptosis and preventing tumor growth. Knockdown of SQSTM1 in cells inhibits ROS production and reverses ALO-induced cell apoptosis. Furthermore, VPS4A is identified as a direct target of ALO, and the amino acids F153 and D263 of VPS4A are confirmed as the binding sites for ALO. Knockout of VPS4A in H1299 cells demonstrates a similar biological effect as ALO treatment. Additionally, ALO enhances the efficacy of the anti-PD-L1/TGF-β bispecific antibody in inhibiting LLC-derived subcutaneous tumor models. Thus, ALO is first identified as a novel late-stage autophagy inhibitor that triggers tumor cell death by targeting VPS4A.
Keywords: VPS4A; apoptosis; autophagy inhibition; non‐small cell lung cancer; sequestosome‐1.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures



References
-
- Bagherniya M., Butler A. E., Barreto G. E., Sahebkar A., Ageing Res. Rev. 2018, 47, 183. - PubMed
-
- Malhotra J., Jabbour S., Orlick M., Riedlinger G., Guo Y., White E., Aisner J., Cancer Treat. Resear. Communicat. 2019, 21, 100158. - PubMed
-
- Vietri M., Radulovic M., Stenmark H., Nat. Rev. Mol. Cell Bio. 2020, 21, 25. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
