Discovery and Profiling of New Multimodal Phenylglycinamide Derivatives as Potent Antiseizure and Antinociceptive Drug Candidates
- PMID: 39166702
- PMCID: PMC11378297
- DOI: 10.1021/acschemneuro.4c00438
Discovery and Profiling of New Multimodal Phenylglycinamide Derivatives as Potent Antiseizure and Antinociceptive Drug Candidates
Abstract
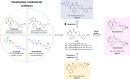
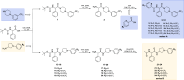
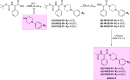
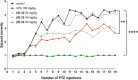
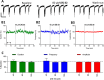
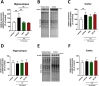
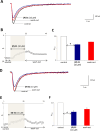
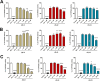
We developed a focused series of original phenyl-glycinamide derivatives which showed potent activity across in vivo mouse seizure models, namely, maximal electroshock (MES) and 6 Hz (using both 32 and 44 mA current intensities) seizure models. Following intraperitoneal (i.p.) administration, compound (R)-32, which was identified as a lead molecule, demonstrated potent protection against all seizure models with ED50 values of 73.9 mg/kg (MES test), 18.8 mg/kg (6 Hz, 32 mA test), and 26.5 mg/kg (6 Hz, 44 mA test). Furthermore, (R)-32 demonstrated efficacy in both the PTZ-induced kindling paradigm and the ivPTZ seizure threshold test. The expression of neurotrophic factors, such as mature brain-derived neurotrophic factor (mBDNF) and nerve growth factor (NGF), in the hippocampus and/or cortex of mice, and the levels of glutamate and GABA were normalized after PTZ-induced kindling by (R)-32. Importantly, besides antiseizure activity, (R)-32 demonstrated potent antinociceptive efficacy in formalin-induced pain, capsaicin-induced pain, as well as oxaliplatin- and streptozotocin-induced peripheral neuropathy in mice (i.p.). No influence on muscular strength and body temperature in mice was observed. Pharmacokinetic studies and in vitro ADME-Tox data (i.e., high metabolic stability in human liver microsomes, a weak influence on CYPs, no hepatotoxicity, satisfactory passive transport, etc.) proved favorable drug-like properties of (R)-32. Thermal stability of (R)-32 shown in thermogravimetry and differential scanning calorimetry gives the opportunity to develop innovative oral solid dosage forms loaded with this compound. The in vitro binding and functional assays indicated its multimodal mechanism of action. (R)-32, beyond TRPV1 antagonism, inhibited calcium and sodium currents at a concentration of 10 μM. Therefore, the data obtained in the current studies justify a more detailed preclinical development of (R)-32 for epilepsy and pain indications.
Keywords: antinociceptive activity; antiseizure activity; hybrid molecules; in vitro ADME-Tox studies; in vitro functional studies; multimechanistic compounds.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

