Comparative Effectiveness of Licensed Influenza Vaccines in Preventing Influenza-related Medical Encounters and Hospitalizations in the 2022-2023 Influenza Season Among Adults ≥65 Years of Age
- PMID: 39166857
- PMCID: PMC11581696
- DOI: 10.1093/cid/ciae375
Comparative Effectiveness of Licensed Influenza Vaccines in Preventing Influenza-related Medical Encounters and Hospitalizations in the 2022-2023 Influenza Season Among Adults ≥65 Years of Age
Abstract
Background: Influenza causes substantial morbidity, particularly among older individuals. Updated data on the effectiveness of currently licensed vaccines in this population are needed.
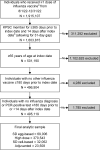
Methods: At Kaiser Permanente Southern California, we conducted a retrospective cohort study to evaluate comparative vaccine effectiveness (cVE) of high-dose (HD), adjuvanted, and standard-dose (SD) cell-based influenza vaccines, relative to the SD egg-based vaccine. We included adults aged ≥65 years who received an influenza vaccine between 1 August 2022 and 31 December 2022, with follow-up up to 20 May 2023. Primary outcomes were: (1) influenza-related medical encounters and (2) polymerase chain reaction (PCR)-confirmed influenza-related hospitalization. Adjusted hazard ratios (aHR) were estimated by Cox proportional hazards regression, adjusting for confounders using inverse probability of treatment weighting (IPTW). cVE (%) was calculated as (1-aHR) × 100 when aHR ≤1, and ([1/aHR]-1) × 100 when aHR >1.
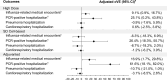
Results: Our study population (n = 495 119) was 54.9% female, 46.3% non-Hispanic White, with a median age of 73 years (interquartile range [IQR] 69-79). Characteristics of all groups were well balanced after IPTW. Adjusted cVEs against influenza-related medical encounters in the HD, adjuvanted, and SD cell-based vaccine groups were 9.1% (95% confidence interval [CI]: .9, 16.7), 16.9% (95% CI: 1.7, 29.8), and -6.3 (95% CI: -18.3, 6.9), respectively. Adjusted cVEs against PCR-confirmed hospitalization in the HD, adjuvanted, and SD cell-based groups were 25.1% (95% CI: .2, 43.8), 61.6% (95% CI: 18.1, 82.0), and 26.4% (95% CI: -18.3, 55.7), respectively.
Conclusions: Compared to the SD egg-based vaccine, HD and adjuvanted vaccines conferred additional protection against influenza-related outcomes in the 2022-2023 season in adults ≥65 years. Our results provide real-world evidence of the comparative effectiveness of currently licensed vaccines.
Keywords: epidemiology; influenza; influenza vaccine; influenza-related medical encounters; vaccine effectiveness.
© The Author(s) 2024. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interests. J. H. K., E. R., L. S. S., L. Q., B. K. A., Y. L., J. E. T., G. S. L., P. P. M., and H. F. T. are employees of Kaiser Permanente Southern California, which has been contracted by Moderna, Inc. to conduct this study. Y. P., T. S., and E. J. A., are employees of and shareholders in Moderna, Inc. J. H. K. received funding from GlaxoSmithKline and Moderna unrelated to this manuscript. E. R. received funding from GlaxoSmithKline unrelated to this manuscript. L. S. S. received funding from GlaxoSmithKline, Dynavax, and Moderna unrelated to this manuscript. L. Q. received funding from GlaxoSmithKline, Dynavax, and Moderna unrelated to this manuscript. B. K. A. received funding from GlaxoSmithKline, Dynavax, Genentech, and Moderna unrelated to this manuscript. Y. L. received funding from GlaxoSmithKline, Pfizer, and Moderna unrelated to this manuscript. J. E. T. received funding from GlaxoSmithKline and Moderna unrelated to this manuscript. G. S. L. received funding from GlaxoSmithKline and Moderna unrelated to this manuscript. P. P. M. received funding from GlaxoSmithKline unrelated to this manuscript. H. F. T. received funding from GlaxoSmithKline and Moderna unrelated to this manuscript; H. F. T. also served on advisory boards for Janssen and Pfizer. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures
References
-
- World Health Organization . Vaccines against influenza: WHO position paper—may 2022. Wkly Epidemiol Rec 2022; 97:186.
-
- Centers for Disease Control and Prevention . Influenza: disease burden of flu. Updated 30 November 2023. Available at: https://www.cdc.gov/flu/about/burden/index.html. Accessed 7 January 2024
-
- Centers for Disease Control and Prevention . Estimated flu-related illnesses, medical visits, hospitalizations, and deaths in the United States—2017–2018 flu season. Available at: https://archive.cdc.gov/#/details?url=https://www.cdc.gov/flu/about/burd.... Accessed 18 July 2023.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

