Use of frozen native feces for fecal microbiota transplantation in recurrent Clostridioides difficile infection: a simple way to improve the efficiency of donor feces preparation
- PMID: 39166867
- PMCID: PMC11459919
- DOI: 10.1128/aac.00734-24
Use of frozen native feces for fecal microbiota transplantation in recurrent Clostridioides difficile infection: a simple way to improve the efficiency of donor feces preparation
Abstract
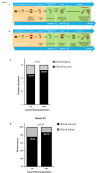
Preparing fecal microbiota transplants immediately after donation is resource-intensive, and a proportion are destroyed following abnormal screening results. We retrospectively compared two processes, frozen fecal preparation (FFP) and fresh native frozen preparation (FNFP), for clinical efficacy in the treatment of recurrent Clostridioides difficile infection (rCDI). FFP and FNFP were similarly effective with clinical success rates of 76.7% and 86.7% (P = 0.32), respectively. FNFP is an efficient procedure that saves resources while maintaining clinical efficacy in rCDI.
Keywords: Clostridium difficile; FMT; fecal microbiota transplantation.
Conflict of interest statement
H.S. reports lecture fee, board membership, or consultancy from Amgen, Fresenius, IPSEN, Actial, Astellas, Danone, THAC, Biose, BiomX, Eligo, Immusmol, Adare, Nestle, Ferring, MSD, Bledina, Pfizer, Biocodex, BMS, Bromatech, Gilead, Janssen, Mayoli, Roche, Sanofi, Servier, Takeda, Abbvie, and Lilly, has stocks from Enterome bioscience, and is co-founder of Exeliom Biosciences.
Figures
References
-
- van Nood E, Vrieze A, Nieuwdorp M, Fuentes S, Zoetendal EG, de Vos WM, Visser CE, Kuijper EJ, Bartelsman J, Tijssen JGP, Speelman P, Dijkgraaf MGW, Keller JJ. 2013. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med 368:407–415. doi:10.1056/NEJMoa1205037 - DOI - PubMed
-
- Ansm . 2016. La transplantation de microbiote fécal et son encadrement dans les essais cliniques
-
- Trang C, Scanzi J, Galperine T, Mosca A, Batista R, Sokol H. 2017. Transplantation de microbiote fécal dans le cadre des infections à Clostridium difficile récidivantes: actualisation des recommandations pour la pratique clinique courante. Hepato-Gastro Oncol Dig 24:319–325. doi:10.1684/hpg.2017.1438 - DOI
-
- Youngster I, Sauk J, Pindar C, Wilson RG, Kaplan JL, Smith MB, Alm EJ, Gevers D, Russell GH, Hohmann EL. 2014. Fecal microbiota transplant for relapsing Clostridium difficile infection using a frozen inoculum from unrelated donors: a randomized, open-label, controlled pilot study. Clin Infect Dis 58:1515–1522. doi:10.1093/cid/ciu135 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical