Pangenomic landscapes shape performances of a synthetic genetic circuit across Stutzerimonas species
- PMID: 39166875
- PMCID: PMC11406997
- DOI: 10.1128/msystems.00849-24
Pangenomic landscapes shape performances of a synthetic genetic circuit across Stutzerimonas species
Abstract
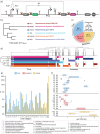
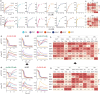
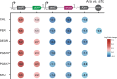
Engineering identical genetic circuits into different species typically results in large differences in performance due to the unique cellular environmental context of each host, a phenomenon known as the "chassis-effect" or "context-dependency". A better understanding of how genomic and physiological contexts underpin the chassis-effect will improve biodesign strategies across diverse microorganisms. Here, we combined a pangenomic-based gene expression analysis with quantitative measurements of performance from an engineered genetic inverter device to uncover how genome structure and function relate to the observed chassis-effect across six closely related Stutzerimonas hosts. Our results reveal that genome architecture underpins divergent responses between our chosen non-model bacterial hosts to the engineered device. Specifically, differential expression of the core genome, gene clusters shared between all hosts, was found to be the main source of significant concordance to the observed differential genetic device performance, whereas specialty genes from respective accessory genomes were not significant. A data-driven investigation revealed that genes involved in denitrification and components of trans-membrane transporter proteins were among the most differentially expressed gene clusters between hosts in response to the genetic device. Our results show that the chassis-effect can be traced along differences among the most conserved genome-encoded functions and that these differences create a unique biodesign space among closely related species.IMPORTANCEContemporary synthetic biology endeavors often default to a handful of model organisms to host their engineered systems. Model organisms such as Escherichia coli serve as attractive hosts due to their tractability but do not necessarily provide the ideal environment to optimize performance. As more novel microbes are domesticated for use as biotechnology platforms, synthetic biologists are urged to explore the chassis-design space to optimize their systems and deliver on the promises of synthetic biology. The consequences of the chassis-effect will therefore only become more relevant as the field of biodesign grows. In our work, we demonstrate that the performance of a genetic device is highly dependent on the host environment it operates within, promoting the notion that the chassis can be considered a design variable to tune circuit function. Importantly, our results unveil that the chassis-effect can be traced along similarities in genome architecture, specifically the shared core genome. Our study advocates for the exploration of the chassis-design space and is a step forward to empowering synthetic biologists with knowledge for more efficient exploration of the chassis-design space to enable the next generation of broad-host-range synthetic biology.
Keywords: biodesign; broad-host-range; chassis-effect; context dependence; genetic inverter; host-circuit interactions; non-model organism; procrustean superimposition; synthetic biology; transcriptomics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Khan N, Yeung E, Farris Y, Fansler SJ, Bernstein HC. 2020. A broad-host-range event detector: expanding and quantifying performance between Escherichia coli and Pseudomonas species. Synth Biol 5:ysaa002. doi: 10.1093/synbio/ysaa002 - DOI
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

