Cyclic-di-GMP induces inflammation and acute lung injury through direct binding to MD2
- PMID: 39166890
- PMCID: PMC11337466
- DOI: 10.1002/ctm2.1744
Cyclic-di-GMP induces inflammation and acute lung injury through direct binding to MD2
Abstract
Background: Severe bacterial infections can trigger acute lung injury (ALI) and acute respiratory distress syndrome, with bacterial pathogen-associated molecular patterns (PAMPs) exacerbating the inflammatory response, particularly in COVID-19 patients. Cyclic-di-GMP (CDG), one of the PAMPs, is synthesized by various Gram-positve and Gram-negative bacteria. Previous studies mainly focused on the inflammatory responses triggered by intracellular bacteria-released CDG. However, how extracellular CDG, which is released by bacterial autolysis or rupture, activates the inflammatory response remains unclear.
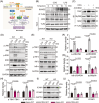
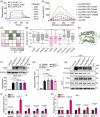
Methods: The interaction between extracellular CDG and myeloid differentiation protein 2 (MD2) was investigated using in vivo and in vitro models. MD2 blockade was achieved using specific inhibitor and genetic knockout mice. Site-directed mutagenesis, co-immunoprecipitation, SPR and Bis-ANS displacement assays were used to identify the potential binding sites of MD2 on CDG.
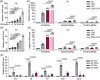
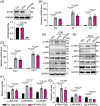
Results: Our data show that extracellular CDG directly interacts with MD2, leading to activation of the TLR4 signalling pathway and lung injury. Specific inhibitors or genetic knockout of MD2 in mice significantly alleviated CDG-induced lung injury. Moreover, isoleucine residues at positions 80 and 94, along with phenylalanine at position 121, are essential for the binding of MD2 to CDG.
Conclusion: These results reveal that extracellular CDG induces lung injury through direct interaction with MD2 and activation of the TLR4 signalling pathway, providing valuable insights into bacteria-induced ALI mechanisms and new therapeutic approaches for the treatment of bacterial co-infection in COVID-19 patients.
Keywords: COVID‐19; MD2; acute lung injury; cyclic‐di‐GMP.
© 2024 The Author(s). Clinical and Translational Medicine published by John Wiley & Sons Australia, Ltd on behalf of Shanghai Institute of Clinical Bioinformatics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Chalcone derivatives ameliorate lipopolysaccharide-induced acute lung injury and inflammation by targeting MD2.Acta Pharmacol Sin. 2022 Jan;43(1):76-85. doi: 10.1038/s41401-021-00764-8. Epub 2021 Sep 3. Acta Pharmacol Sin. 2022. PMID: 34480112 Free PMC article.
-
Inhibition of myeloid differentiation factor 2 by baicalein protects against acute lung injury.Phytomedicine. 2019 Oct;63:152997. doi: 10.1016/j.phymed.2019.152997. Epub 2019 Jun 20. Phytomedicine. 2019. PMID: 31254764
-
Extracellular peroxiredoxin 6 released from alveolar epithelial cells as a DAMP drives macrophage activation and inflammatory exacerbation in acute lung injury.Int Immunopharmacol. 2025 Feb 20;148:114023. doi: 10.1016/j.intimp.2025.114023. Epub 2025 Jan 16. Int Immunopharmacol. 2025. PMID: 39823791
-
Structural Diversity and Mutational Challenges of Toll-Like Receptor 4 Antagonists as Inflammatory Pathway Blocker.Drug Dev Res. 2025 Feb;86(1):e70031. doi: 10.1002/ddr.70031. Drug Dev Res. 2025. PMID: 39690962 Review.
-
Recent progress in the discovery of myeloid differentiation 2 (MD2) modulators for inflammatory diseases.Drug Discov Today. 2018 Jun;23(6):1187-1202. doi: 10.1016/j.drudis.2018.01.015. Epub 2018 Jan 9. Drug Discov Today. 2018. PMID: 29330126 Review.
Cited by
-
Targeting myeloid differentiation protein 2 ameliorates rheumatoid arthritis by inhibiting inflammation and ferroptosis via MAPK and NF-κB signaling pathways.J Mol Med (Berl). 2025 Jul;103(7):821-836. doi: 10.1007/s00109-025-02555-8. Epub 2025 May 21. J Mol Med (Berl). 2025. PMID: 40397030
References
-
- Zhu W, Wang M, Jin L, et al. Licochalcone A protects against LPS‐induced inflammation and acute lung injury by directly binding with myeloid differentiation factor 2 (MD2). Br J Pharmacol. 2023;180(8):1114‐1131. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
