Optimized methods for measuring competitive binding of chemical substances to thyroid hormone distributor proteins transthyretin and thyroxine binding globulin
- PMID: 39167138
- PMCID: PMC11489250
- DOI: 10.1007/s00204-024-03842-y
Optimized methods for measuring competitive binding of chemical substances to thyroid hormone distributor proteins transthyretin and thyroxine binding globulin
Abstract
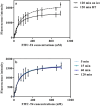
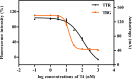
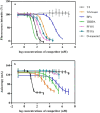
Transthyretin (TTR) and thyroxine-binding globulin (TBG) are two major thyroid hormone (TH) distributor proteins in human plasma, playing important roles in stabilizing the TH levels in plasma, delivery of TH to target tissues, and trans-barrier transport. Binding of xenobiotics to these distributor proteins can potentially affect all these three important roles of distributor proteins. Therefore, fast and cost-effective experimental methods are required for both TTR and TBG to screen both existing and new chemicals for their potential binding. In the present study, the TTR-binding assay was therefore simplified, optimized and pre-validated, while a new TBG-binding assay was developed based on fluorescence polarization as a readout. Seven model compounds (including positive and negative controls) were tested in the pre-validation study of the optimized TTR-binding assay and in the newly developed TBG-binding assay. The dissociation constants of the natural ligand (thyroxine, T4) and potential competitors were determined and compared between two distributor proteins, showing striking differences for perfluorooctanesulfonic acid (PFOS) and perfluorooctanoic acid (PFOA).
Keywords: Fluorescence polarization; Per- and polyfluoroalkyl substances; TBG binding; TTR binding; Thyroid hormone system disruptors.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Alshehri B, D’Souza DG, Lee JY, Petratos S, Richardson SJ (2015) The diversity of mechanisms influenced by transthyretin in neurobiology: development, disease and endocrine disruption. J Neuroendocrinol 27(5):303–323 - PubMed
-
- Bernasconi C, Langezaal I, Bartnicka J, Asturiol D, Bowe G, Coecke S, Kienzler A, Liska R, Milcamps A, Munoz-Pineiro MA, Pistollato F and Whelan M (2023) Validation of a battery of mechanistic methods relevant for the detection of chemicals that can disrupt the thyroid hormone system. In. https://publications.jrc.ec.europa.eu/repository/handle/JRC132532. Accessed 30-Nov 2023
-
- Boas M, Feldt-Rasmussen U, Skakkebæk NE, Main KM (2006) Environmental chemicals and thyroid function. Eur J Endocrinol 154(5):599–611 - PubMed
-
- Cao J, Lin Y, Guo L-H, Zhang A-Q, Wei Y, Yang Y (2010) Structure-based investigation on the binding interaction of hydroxylated polybrominated diphenyl ethers with thyroxine transport proteins. Toxicology 277(1–3):20–28 - PubMed
-
- Cao J, Guo L-H, Wan B, Wei Y (2011) In vitro fluorescence displacement investigation of thyroxine transport disruption by bisphenol A. J Environ Sci 23(2):315–321 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

