Redefining Cardiac Involvement and Targets of Treatment in Systemic Immunoglobulin AL Amyloidosis
- PMID: 39167388
- PMCID: PMC11339700
- DOI: 10.1001/jamacardio.2024.2555
Redefining Cardiac Involvement and Targets of Treatment in Systemic Immunoglobulin AL Amyloidosis
Abstract
Importance: Cardiac amyloid infiltration is the key determinant of survival in systemic light-chain (AL) amyloidosis. Current guidelines recommend early switching therapy in patients with a nonoptimal or suboptimal response regardless of the extent of cardiac amyloid infiltration.
Objective: To assess the differences between serum biomarkers, echocardiography, and cardiovascular magnetic resonance (CMR) with extracellular volume (ECV) mapping in characterizing cardiac amyloid, the independent prognostic role of these approaches, and the role of ECV mapping to guide treatment strategies.
Design, setting, and participants: Consecutive patients newly diagnosed with systemic AL amyloidosis (2015-2021) underwent echocardiography, cardiac biomarkers, and CMR with ECV mapping at diagnosis. Data were analyzed from January to June 2024.
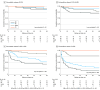
Main outcomes and measures: The primary outcomes of the study were all-cause mortality and hematological response as defined according to validated criteria: no response (NR), partial response (PR), very good partial response (VGPR), and complete response (CR). Secondary outcomes were the depth and speed of hematological response and overall survival according to ECV.
Results: Of 560 patients with AL amyloidosis, the median (IQR) age was 68 years (59-74 years); 346 patients were male (61.8%) and 214 female (38.2%). Over a median (IQR) 40.5 months 9-58 months), ECV was independently associated with mortality. In the landmark analysis at 1 month, long-term survival was independent of the achieved hematological response in ECV less than 0.30% and ECV of 0.31% to 0.40%, while it was dependent on the depth of the hematological response in ECV greater than 0.40%. In the landmark analysis at 6 months, survival was independent of the achieved hematological response in ECV less than 0.30% and dependent on achieving at least PR in ECV of 0.31% to 0.40%. Survival was dependent on achieving CR in ECV of 0.41% to 0.50% and ECV greater than 0.50%. Achieving a deep hematological response at 1 month was associated with better survival compared with 6 months in patients with ECV greater than 0.40% but not with ECV less than 0.40%.
Conclusions and relevance: This study found that ECV mapping, in systemic AL amyloidosis, is an independent predictor of prognosis, can help define the hematological response associated with better long-term outcomes for each patient and potentially inform treatment strategies.
Conflict of interest statement
Figures

References
-
- Gertz MA, Comenzo R, Falk RH, et al. . Definition of organ involvement and treatment response in immunoglobulin light chain amyloidosis (AL): a consensus opinion from the 10th International Symposium on Amyloid and Amyloidosis, Tours, France, 18-22 April 2004. Am J Hematol. 2005;79(4):319-328. doi:10.1002/ajh.20381 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

