The microbiota-dependent tryptophan metabolite alleviates high-fat diet-induced insulin resistance through the hepatic AhR/TSC2/mTORC1 axis
- PMID: 39167602
- PMCID: PMC11363250
- DOI: 10.1073/pnas.2400385121
The microbiota-dependent tryptophan metabolite alleviates high-fat diet-induced insulin resistance through the hepatic AhR/TSC2/mTORC1 axis
Abstract
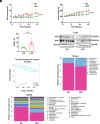
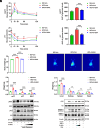
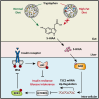
Type 2 diabetes (T2D) is potentially linked to disordered tryptophan metabolism that attributes to the intricate interplay among diet, gut microbiota, and host physiology. However, underlying mechanisms are substantially unknown. Comparing the gut microbiome and metabolome differences in mice fed a normal diet (ND) and high-fat diet (HFD), we uncover that the gut microbiota-dependent tryptophan metabolite 5-hydroxyindole-3-acetic acid (5-HIAA) is present at lower concentrations in mice with versus without insulin resistance. We further demonstrate that the microbial transformation of tryptophan into 5-HIAA is mediated by Burkholderia spp. Additionally, we show that the administration of 5-HIAA improves glucose intolerance and obesity in HFD-fed mice, while preserving hepatic insulin sensitivity. Mechanistically, 5-HIAA promotes hepatic insulin signaling by directly activating AhR, which stimulates TSC2 transcription and thus inhibits mTORC1 signaling. Moreover, T2D patients exhibit decreased fecal levels of 5-HIAA. Our findings identify a noncanonical pathway of microbially producing 5-HIAA from tryptophan and indicate that 5-HIAA might alleviate the pathogenesis of T2D.
Keywords: gut microbiota; insulin signaling; tryptophan metabolism; type 2 diabetes.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures




References
-
- Aurelie C., et al. , Dietary intervention impact on gut microbial gene richness. Nature 500, 585–588 (2013). - PubMed
-
- Chatelier E.-L., et al. , Richness of human gut microbiome correlates with metabolic markers. Nature 500, 541–546 (2013). - PubMed
-
- Qin J., et al. , A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 490, 55–60 (2012). - PubMed
-
- Brown M.-S., Goldstein J.-L., Selective versus total insulin resistance: A pathogenic paradox. Cell Metab. 7, 95–96 (2008). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

