Pembrolizumab and Cabozantinib in Recurrent and/or Metastatic Head and Neck Squamous Cell Carcinoma: Long-term Survival Update with a Biomarker Analysis
- PMID: 39167623
- PMCID: PMC11479816
- DOI: 10.1158/1078-0432.CCR-24-1202
Pembrolizumab and Cabozantinib in Recurrent and/or Metastatic Head and Neck Squamous Cell Carcinoma: Long-term Survival Update with a Biomarker Analysis
Abstract
Purpose: Anti-programmed cell death protein 1 (PD-1) therapy is a standard of care in recurrent and/or metastatic head and neck squamous cell carcinoma (RMHNSCC). Vascular endothelial growth factor receptor tyrosine kinase inhibitors (VEGFR-TKI) have immunomodulatory properties and improve clinical outcomes in combination with anti-PD-1 therapy in different malignancies. We report the long-term efficacy and safety of pembrolizumab and cabozantinib in patients with RMHNSCC and include a correlative biomarker analysis.
Patients and methods: This open-label, single-arm, multicenter, phase 2 study screened 50 patients with RMHNSCC, of whom 36 received pembrolizumab and cabozantinib. The primary endpoint was overall response rate (ORR), safety, and tolerability. Secondary endpoints included progression-free survival (PFS), overall survival (OS), and correlative studies of tissue and blood. We report the long-term PFS, OS, and safety of treated patients and describe correlative biomarkers evaluating p-MET expression and tumor immune microenvironment (TIME) using multiplex immunohistochemistry.
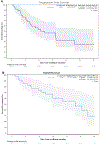
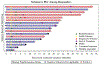
Results: With median follow-up of 22.4 months, the median PFS was 12.8 months with a 2-year PFS of 32.6% (95% CI, 18.8%-56.3%) and the median OS was 27.7 months with a 2-year OS of 54.7% [95% confidence interval (CI), 38.9%-76.8%]. The median duration of response was 12.6 months with a 2-year rate of 38.5% (95% CI, 30.8%-81.8%). Long-term treatment-related adverse events included manageable hypothyroidism (5.5%) and grade 1 elevated aspartate aminotransferase and alanine aminotransferase (2.8%). Baseline tumor p-MET expression correlated with ORR (P = 0.0055). Higher density of CD8+, CD103+, and CSF1-R+ cells at baseline correlated with improved OS [hazard ratio (HR) = 5.27, P = 0.030; HR = 8.79, P = 0.017; HR = 6.87, P = 0.040, respectively].
Conclusions: Pembrolizumab and cabozantinib provided prolonged encouraging long-term disease control and survival with a maintained favorable safety profile. The prognostic significance of higher density of CD8+, CD103+, and CSF1-R+ cells in TIME deserve further evaluation in similar clinical settings.
©2024 American Association for Cancer Research.
Conflict of interest statement
Potential Conflict of Interest:
NFS- reports advisory role with or without compensation from Astra Zeneca, Eisai Medical, Exelixis, Merck, EMD Serono, Pfizer, Kura, Vaccinex, CUE, BionTech, GSK, TOSK, Seagen, Flamingo, Infinity, Inovio, Aveo, BMS, Cornerstone, Celldex, Surface Oncology, Astex, Imugene, Faron Pharmaceutical, Coherus, Adagene, Fulgent, Nanobiotix, Taiho
CHC and RJCS—honoraria from Fulgent, Genmab, AVEO, Seagen, Regeneron, and Exelixis for ad hoc Scientific Advisory Board participation.
JEB – ad hoc scientific advisory board participation for Galera Therapeutics and Castle Biosciences
Figures


Comment in
-
Novel combinations with programmed cell death 1 inhibitor for incurable recurrent/metastatic head and neck squamous cell carcinoma (RM HNSCC): is cabozantinib a front runner?Transl Cancer Res. 2025 May 30;14(5):2548-2552. doi: 10.21037/tcr-2024-2569. Epub 2025 May 13. Transl Cancer Res. 2025. PMID: 40530149 Free PMC article. No abstract available.
References
-
- Burtness B, Harrington KJ, Greil R, Soulieres D, Tahara M, de Castro G Jr., et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet 2019;394(10212):1915–28 doi 10.1016/S0140-6736(19)32591-7. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

