Cellular stiffness sensing through talin 1 in tissue mechanical homeostasis
- PMID: 39167642
- PMCID: PMC11338229
- DOI: 10.1126/sciadv.adi6286
Cellular stiffness sensing through talin 1 in tissue mechanical homeostasis
Abstract
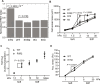
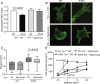
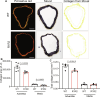
Tissue mechanical properties are determined mainly by the extracellular matrix (ECM) and actively maintained by resident cells. Despite its broad importance to biology and medicine, tissue mechanical homeostasis remains poorly understood. To explore cell-mediated control of tissue stiffness, we developed mutations in the mechanosensitive protein talin 1 to alter cellular sensing of ECM. Mutation of a mechanosensitive site between talin 1 rod-domain helix bundles R1 and R2 increased cell spreading and tension exertion on compliant substrates. These mutations promote binding of the ARP2/3 complex subunit ARPC5L, which mediates the change in substrate stiffness sensing. Ascending aortas from mice bearing these mutations showed less fibrillar collagen, reduced axial stiffness, and lower rupture pressure. Together, these results demonstrate that cellular stiffness sensing contributes to ECM mechanics, directly supporting the mechanical homeostasis hypothesis and identifying a mechanosensitive interaction within talin that contributes to this mechanism.
Figures





Update of
-
Mechanosensing through talin 1 contributes to tissue mechanical homeostasis.bioRxiv [Preprint]. 2024 Jan 26:2023.09.03.556084. doi: 10.1101/2023.09.03.556084. bioRxiv. 2024. Update in: Sci Adv. 2024 Aug 23;10(34):eadi6286. doi: 10.1126/sciadv.adi6286. PMID: 38328095 Free PMC article. Updated. Preprint.
References
-
- Wang Y., Gao P., Li F., Du J., Insights on aortic aneurysm and dissection: Role of the extracellular environment in vascular homeostasis. J. Mol. Cell. Cardiol. 171, 90–101 (2022). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

