Role of bacteriophages in shaping gut microbial community
- PMID: 39167701
- PMCID: PMC11340752
- DOI: 10.1080/19490976.2024.2390720
Role of bacteriophages in shaping gut microbial community
Abstract
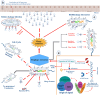
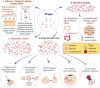
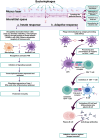
Phages are the most diversified and dominant members of the gut virobiota. They play a crucial role in shaping the structure and function of the gut microbial community and consequently the health of humans and animals. Phages are found mainly in the mucus, from where they can translocate to the intestinal organs and act as a modulator of gut microbiota. Understanding the vital role of phages in regulating the composition of intestinal microbiota and influencing human and animal health is an emerging area of research. The relevance of phages in the gut ecosystem is supported by substantial evidence, but the importance of phages in shaping the gut microbiota remains unclear. Although information regarding general phage ecology and development has accumulated, detailed knowledge on phage-gut microbe and phage-human interactions is lacking, and the information on the effects of phage therapy in humans remains ambiguous. In this review, we systematically assess the existing data on the structure and ecology of phages in the human and animal gut environments, their development, possible interaction, and subsequent impact on the gut ecosystem dynamics. We discuss the potential mechanisms of prophage activation and the subsequent modulation of gut bacteria. We also review the link between phages and the immune system to collect evidence on the effect of phages on shaping the gut microbial composition. Our review will improve understanding on the influence of phages in regulating the gut microbiota and the immune system and facilitate the development of phage-based therapies for maintaining a healthy and balanced gut microbiota.
Keywords: Bacteriophage; antimicrobials; gut microbiota modulation; gut virobiota; modulation of gut metabolites; phage and human immune system; phage therapy; prophage activation.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
