Influence of Different Diagnostic Criteria on Alzheimer Disease Clinical Research
- PMID: 39167736
- PMCID: PMC11338500
- DOI: 10.1212/WNL.0000000000209753
Influence of Different Diagnostic Criteria on Alzheimer Disease Clinical Research
Abstract
Background and objectives: Updates in Alzheimer disease (AD) diagnostic guidelines by the National Institute on Aging-Alzheimer's Association (NIA-AA) and the International Working Group (IWG) over the past 11 years may affect clinical diagnoses. We assessed how these guidelines affect clinical AD diagnosis in a cohort of cognitively unimpaired (CU) and cognitively impaired (CI) individuals.
Methods: We applied clinical and biomarker data in algorithms to classify individuals from the Alzheimer's Disease Neuroimaging Initiative (ADNI) cohort according to the following diagnostic guidelines for AD: 2011 NIA-AA, 2016 IWG-2, 2018 NIA-AA, and 2021 IWG-3, assigning the following generic diagnostic labels: (1) not AD (nAD), (2) increased risk of developing AD (irAD), and (3) AD. Diagnostic labels were compared according to their frequency, convergence across guidelines, biomarker profiles, and prognostic value. We also evaluated the diagnostic discordance among the criteria.
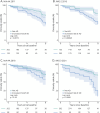
Results: A total of 1,195 individuals (mean age 73.2 ± 7.2 years, mean education 16.1 ± 2.7, 44.0% female) presented different repartitions of diagnostic labels according to the 2011 NIA-AA (nAD = 37.8%, irAD = 23.0%, AD = 39.2%), 2016 IWG-2 (nAD = 37.7%, irAD = 28.7%, AD = 33.6%), 2018 NIA-AA (nAD = 40.7%, irAD = 9.3%, AD = 50.0%), and 2021 IWG-3 (nAD = 51.2%, irAD = 8.4%, AD = 48.3%) frameworks. Discordant diagnoses across all guidelines were found in 512 participants (42.8%) (138 [91.4%] occurring in only β-amyloid [CU 65.4%, CI 34.6%] and 191 [78.6%] in only tau-positive [CU 71.7%, CI 28.3%] individuals). Differences in predicting cognitive impairment between nAD and irAD groups were observed with the 2011 NIA-AA (hazard ratio [HR] 2.21, 95% CI 1.34-3.65, p = 0.002), 2016 IWG-2 (HR 2.81, 95% CI 1.59-4.96, p < 0.000), and 2021 IWG-3 (HR 3.61, 95% CI 2.09-6.23, p < 0.000), but not with 2018 NIA-AA (HR 1.69, 95% CI 0.87-3.28, p = 0.115).
Discussion: Over 42% of the studied population presented discordant diagnoses when using the different examined AD criteria, mostly in individuals with a single positive biomarker. Except for 2018 NIA-AA, all guidelines identified asymptomatic individuals at risk of cognitive impairment. Our findings highlight important differences between the guidelines, emphasizing the necessity for updated criteria with enhanced staging metrics, considering clinical, research, therapeutic, and trial design aspects.
Conflict of interest statement
A. Bieger is a cofounder and a minority shareholder at masima and received financial support from CAPES (88887.627297/2021-00). W.S. Brum is supported by CAPES (88887.372371/2019-00 and 88887.596742/2020-00) and Stiftelsen för GamlaTjänarinnor. W.J.V. Borelli is a cofounder and a minority shareholder at masima. M.A. de Bastiani is a cofounder and a minority shareholder at masima. J.P. Ferrari-Souza receives financial support from CNPq (200691/2021-0). D.O. Souza is supported by CNPq/INCT (465671/2014-4), CNPq/FAPERGS/PRONEX (16/2551-0000475-7), and FAPERGS (19/2551-0000700-0). R.M. Castilhos receives financial support from the Alzheimer's Association (AARGD-21-846545). M. Schöll is supported by the Knut and Alice Wallenberg Foundation (Wallenberg Centre for Molecular and Translational Medicine Fellow; KAW2014.0363), the Swedish Research Council (2017-02869, 2021-02678 and 2021-06545), the Swedish state under the agreement between the Swedish government and the County Councils, the ALF-agreement (ALFGBG-813971 and ALFGBG-965326), the Swedish Brain Foundation (FO2021-0311) and the Swedish Alzheimer Foundation (AF-740191). H. Zetterberg is a Wallenberg Scholar supported by grants from the Swedish Research Council (2018-02532), the European Research Council (681712 and 101053962), Swedish State Support for Clinical Research (ALFGBG-71320), the Alzheimer Drug Discovery Foundation (ADDF), USA (201809-2016862), the AD Strategic Fund and the Alzheimer's Association (ADSF-21-831376-C, ADSF-21-831381-C, and ADSF-21-831377-C), the Olav Thon Foundation, the Erling-Persson Family Foundation, Stiftelsen för GamlaTjänarinnor, Hjärnfonden, Sweden (FO2019-0228), the European Union's Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 860197 (MIRIADE), the European Union Joint Programme—Neurodegenerative Disease Research (JPND2021-00694), and the UK Dementia Research Institute at UCL (UKDRI-1003), has served at scientific advisory boards and/or as a consultant for Abbvie, Alector, Annexon, Apellis, Artery Therapeutics, AZTherapies, CogRx, Denali, Eisai, Nervgen, Novo Nordisk, Pinteon Therapeutics, Red Abbey Labs, Passage Bio, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics, and Wave, has given lectures in symposia sponsored by Cellectricon, Fujirebio, Alzecure, Biogen, and Roche, and is a cofounder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work). K. Blennow is supported by the Swedish Research Council (2017-00915), the Alzheimer Drug Discovery Foundation (ADDF), USA (RDAPB-201809-2016615), the Swedish Alzheimer Foundation (AF-930351, AF-939721, and AF-968270), Hjärnfonden, Sweden (FO2017-0243 and ALZ2022-0006), the Swedish state under the agreement between the Swedish government and the County Councils, the ALF-agreement (ALFGBG-715986 and ALFGBG-965240), the European Union Joint Program for Neurodegenerative Disorders (JPND2019-466-236), the National Institute of Health (NIH), USA (1R01AG068398-01), and the Alzheimer's Association 2021 Zenith Award (ZEN-21-848495), has served as a consultant and at advisory boards for Acumen, ALZPath, AriBio, BioArctic, Biogen, Eisai, Lilly, Moleac Pte. Ltd., Novartis, Ono Pharma, Prothena, Roche Diagnostics, and Siemens Healthineers, has served at data monitoring committees for Julius Clinical and Novartis, has given lectures, produced educational materials and participated in educational programs for AC Immune, Biogen, Celdara Medical, Eisai, and Roche Diagnostics; and is a cofounder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program, outside the work presented in this paper. P. Rosa-Neto receives financial support from Weston Brain Institute, Canadian Institutes of Health Research (CIHR) (MOP-11-51-31, FRN, 152985, PI:PR-N), Salary Award Fonds de Recherche du Québec—Santé (FRQS), and the Colin J. Adair Charitable Foundation, has served on scientific advisory boards for Novonordisk, Eisai and Eli Lilly, and has served as a consultant to Eisai and Cerveau Radiopharmaceuticals. E.R. Zimmer receives financial support from CNPq (312410/2018-2, 435642/2018-9, 409066/2022-2, and 312306/2021-0), ARD/FAPERGS (21/2551-0000673-0), the Alzheimer's Association (AARGD-21-850670), PRONEX, FAPERGS/CNPq (16/2551-0000475-7), the Brazilian National Institute of Science and Technology in Excitotoxicity and Neuroprotection (465671/2014-4), Instituto Serrapilheira (Serra-1912-31365), and the Alzheimer's Association and National Academy of Neuropsycology (ALZ-NAN-22-928381), has served in the scientific advisory boards of Nintx, Novo Nordisk, and masima, and is also a cofounder and a minority shareholder at masima. All other authors report no relevant disclosures. Go to
Figures


References
-
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34(7):939-944. doi: 10.1212/wnl.34.7.939 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
