HSD3B1 genotype and outcomes in metastatic hormone-sensitive prostate cancer with androgen deprivation therapy and enzalutamide: ARCHES
- PMID: 39168093
- PMCID: PMC11384952
- DOI: 10.1016/j.xcrm.2024.101644
HSD3B1 genotype and outcomes in metastatic hormone-sensitive prostate cancer with androgen deprivation therapy and enzalutamide: ARCHES
Abstract
HSD3B1 encodes 3β-hydroxysteroid dehydrogenase-1, which converts adrenal dehydroepiandrosterone to 5α-dihydrotestosterone and is inherited in adrenal-permissive (AP) or adrenal-restrictive forms. The AP allele is linked to castration resistance, mainly in low-volume tumors. Here, we investigate the association of HSD3B1 alleles with outcomes in ARCHES, a multinational, double-blind, randomized, placebo-controlled phase 3 trial that demonstrated clinical benefit with enzalutamide plus androgen deprivation therapy (ADT) in men with metastatic hormone-sensitive prostate cancer (mHSPC) compared to those treated with placebo plus ADT. There are no significant differences between genotypes for clinical efficacy endpoints. Enzalutamide significantly improves radiographic progression-free survival and overall survival vs. placebo irrespective of HSD3B1 status. Men with the AP genotype have higher post-progression mortality and treatment-emergent adverse events, including hypertension, cardiovascular events, and gynecomastia, but a lower fracture rate. Overall, enzalutamide is beneficial in men with mHSPC independent of the HSD3B1 genotype. Inherited polymorphisms of HSD3B1 may account for differential toxicities.
Keywords: HSD3B1; androgens; enzalutamide; metabolism; metastatic hormone-sensitive prostate cancer.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests N.S. reports consulting for Celgene and Roivant, research funding from Astellas, and a filed patent application by Cleveland Clinic for a method of steroid-dependent disease treatment based on HSD3B1. A.A.A. reports honoraria from Aculeus Therapeutics, Amgen, Astellas, AstraZeneca, Bayer, Bristol Myers Squibb, Ipsen, Janssen, Merck, Merck Serono, Novartis, Noxopharm, Pfizer, Sanofi, Telix, and Tolmar; consulting for Aculeus Therapeutics, Amgen, Astellas, AstraZeneca, Bayer, Bristol Myers Squibb, Ipsen, Janssen, Merck, Merck Serono, Novartis, Noxopharm, Pfizer, Sanofi, Telix, and Tolmar; speakers’ bureau for Amgen, Astellas, Bayer, Bristol Myers Squibb, Ipsen, Janssen, Merck Serono, and Novartis; grants from Aptevo (institutional), Astellas (institutional), Astellas (investigator), AstraZeneca (institutional), AstraZeneca (investigator), Bionomics (institutional), Bristol Myers Squibb (institutional), Exelixis (institutional), Gilead Sciences (institutional), GlaxoSmithKline (institutional), Hinova (institutional), Ipsen (institutional), Janssen (institutional), Lilly (institutional), MedImmune (institutional), Merck Serono (institutional), Merck Serono (investigator), Merck Sharpe & Dohme (institutional), Novartis (institutional), Pfizer (institutional), Sanofi Aventis (institutional), and Synthorx (institutional); travel/accommodation reimbursement from Amgen, Astellas, Bayer, Janssen, Merck Serono, Pfizer, Sanofi, and Tolmar; participation on an advisory board for Amgen, Astellas, AstraZeneca, Bayer, Bristol Myers Squibb, Ipsen, Janssen, Merck, Merck Serono, Novartis, Noxopharm, Pfizer, Sanofi, Telix, and Tolmar; and participation as the chair of the Urologic Oncology Group for the Clinical Oncology Society of Australia and as a chair of the Translational Research Subcommittee and member of the Scientific Advisory Committee for the ANZUP Cancer Trials Group. J.W.D.H. reports consulting for Astellas. M.W. is an employee of Astellas and reports ownership of stock of ADMA Biologics, CASI, Merck, and Seelos. F.Z. is an employee of Pfizer and reports ownership of stock of Pfizer and AlloVir and patents with AlloVir. J.S. is an employee of Astellas and reports ownership of stock of AstraZeneca. G.P.H. is an employee of Astellas. A.S. reports consulting for Alere, Astellas, Bayer, Bristol Myers Squibb, Ferring, Ipsen, Janssen, Roche, Steba Biotech, and Synergo; research funding from Amgen, Astellas, AstraZeneca, Bayer, Cepheid, GenomeDx, Immatics, Janssen, Johnson & Johnson, Karl Storz, Medivation, Novartis, and Roche; patents for A290/99 implantable incontinence device, AT00/0001:C-Trap implantable device to treat urinary incontinence, and 2018/6579 gene-expression signature for subtype and prognostic prediction of renal cell carcinoma; expert testimony for GBA Pharma; and travel support from Amgen, Astellas, AstraZeneca, CureVac, Ferring, Ipsen, Janssen, and Sanofi/Aventis. A.J.A. reports research support (institutional) to Duke from the NIH/NCI, PCF/Movember, DOD, Astellas, Pfizer, Bayer, Janssen, Dendreon, BMS, AstraZeneca, Merck, Forma, Celgene, Amgen, and Novartis and personal compensation from consulting or advising relationships with Astellas, AstraZeneca, Bayer, Bristol Myers Squibb, Celgene, Clovis, Dendreon, Epic Sciences, Exact Sciences, Exelixis, Forma, GoodRx, Janssen, Merck, Myovant, Novartis, Pfizer, and Point.
Figures


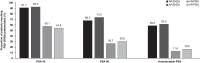

References
-
- Bruchovsky N., Wilson J.D. The conversion of testosterone to 5-alpha-androstan-17-beta-ol-3-one by rat prostate in vivo and in vitro. J. Biol. Chem. 1968;243:2012–2021. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

