Targeting miR-29 mitigates skeletal senescence and bolsters therapeutic potential of mesenchymal stromal cells
- PMID: 39168101
- PMCID: PMC11384963
- DOI: 10.1016/j.xcrm.2024.101665
Targeting miR-29 mitigates skeletal senescence and bolsters therapeutic potential of mesenchymal stromal cells
Abstract
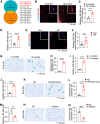
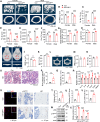
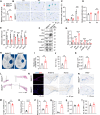
Mesenchymal stromal cell (MSC) senescence is a key factor in skeletal aging, affecting the potential of MSC applications. Identifying targets to prevent MSC and skeletal senescence is crucial. Here, we report increased miR-29 expression in bone tissues of aged mice, osteoporotic patients, and senescent MSCs. Genetic overexpression of miR-29 in Prx1-positive MSCs significantly accelerates skeletal senescence, reducing cortical bone thickness and trabecular bone mass, while increasing femur cross-sectional area, bone marrow adiposity, p53, and senescence-associated secretory phenotype (SASP) levels. Mechanistically, miR-29 promotes senescence by upregulating p53 via targeting Kindlin-2 mRNA. miR-29 knockdown in BMSCs impedes skeletal senescence, enhances bone mass, and accelerates calvarial defect regeneration, also reducing lipopolysaccharide (LPS)-induced organ injuries and mortality. Thus, our findings underscore miR-29 as a promising therapeutic target for senescence-related skeletal diseases and acute inflammation-induced organ damage.
Keywords: Kindlin-2; MSC therapy; aging; bone; bone repair; miR-29; osteoporosis; p53; skeletal senescence.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures





Similar articles
-
miR-203-3p promotes senescence of mouse bone marrow mesenchymal stem cells via downregulation of Pbk.Aging Cell. 2024 Nov;23(11):e14293. doi: 10.1111/acel.14293. Epub 2024 Aug 9. Aging Cell. 2024. PMID: 39123275 Free PMC article.
-
Mesenchymal stem cell-derived small extracellular vesicles mitigate oxidative stress-induced senescence in endothelial cells via regulation of miR-146a/Src.Signal Transduct Target Ther. 2021 Oct 22;6(1):354. doi: 10.1038/s41392-021-00765-3. Signal Transduct Target Ther. 2021. PMID: 34675187 Free PMC article.
-
Human umbilical cord-derived mesenchymal stem cell therapy ameliorates lupus through increasing CD4+ T cell senescence via MiR-199a-5p/Sirt1/p53 axis.Theranostics. 2021 Jan 1;11(2):893-905. doi: 10.7150/thno.48080. eCollection 2021. Theranostics. 2021. PMID: 33391511 Free PMC article.
-
Targeting cellular senescence in kidney diseases and aging: A focus on mesenchymal stem cells and their paracrine factors.Cell Commun Signal. 2024 Dec 18;22(1):609. doi: 10.1186/s12964-024-01968-1. Cell Commun Signal. 2024. PMID: 39696575 Free PMC article. Review.
-
Cellular senescence in bone.Bone. 2019 Apr;121:121-133. doi: 10.1016/j.bone.2019.01.015. Epub 2019 Jan 16. Bone. 2019. PMID: 30659978 Free PMC article. Review.
Cited by
-
The emerging role of miRNAs in biological aging and age-related diseases.Noncoding RNA Res. 2025 May 5;13:131-152. doi: 10.1016/j.ncrna.2025.05.002. eCollection 2025 Aug. Noncoding RNA Res. 2025. PMID: 40501482 Free PMC article. Review.
-
March5-mediated Trim28 degradation preserves islet β-cell function in mice.Nat Commun. 2025 Aug 1;16(1):7073. doi: 10.1038/s41467-025-62587-z. Nat Commun. 2025. PMID: 40750777 Free PMC article.
-
Development of a microRNA-Based age estimation model using whole-blood microRNA expression profiling.Noncoding RNA Res. 2025 Mar 3;12:81-91. doi: 10.1016/j.ncrna.2025.03.003. eCollection 2025 Jun. Noncoding RNA Res. 2025. PMID: 40144340 Free PMC article.
-
Targeting p53-p21 signaling to enhance mesenchymal stem cell regenerative potential.Regen Ther. 2025 Apr 7;29:352-363. doi: 10.1016/j.reth.2025.03.007. eCollection 2025 Jun. Regen Ther. 2025. PMID: 40248767 Free PMC article. Review.
-
Stem cells in the treatment of myocardial injury-induced cardiomyopathy: mechanisms and efficient utilization strategies.Front Pharmacol. 2025 Jun 18;16:1600604. doi: 10.3389/fphar.2025.1600604. eCollection 2025. Front Pharmacol. 2025. PMID: 40606613 Free PMC article. Review.
References
-
- Ramanadham S., Yarasheski K.E., Silva M.J., Wohltmann M., Novack D.V., Christiansen B., Tu X., Zhang S., Lei X., Turk J. Age-related changes in bone morphology are accelerated in group VIA phospholipase A2 (iPLA2beta)-null mice. Am. J. Pathol. 2008;172:868–881. doi: 10.2353/ajpath.2008.070756. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

