Soluble Tim-3 serves as a tumor prognostic marker and therapeutic target for CD8+ T cell exhaustion and anti-PD-1 resistance
- PMID: 39168104
- PMCID: PMC11384939
- DOI: 10.1016/j.xcrm.2024.101686
Soluble Tim-3 serves as a tumor prognostic marker and therapeutic target for CD8+ T cell exhaustion and anti-PD-1 resistance
Abstract
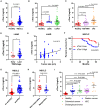
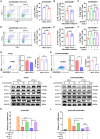
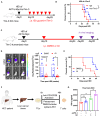
Resistance to PD-1 blockade in onco-immunotherapy greatly limits its clinical application. T cell immunoglobulin and mucin domain containing-3 (Tim-3), a promising immune checkpoint target, is cleaved by ADAM10/17 to produce its soluble form (sTim-3) in humans, potentially becoming involved in anti-PD-1 resistance. Herein, serum sTim-3 upregulation was observed in non-small cell lung cancer (NSCLC) and various digestive tumors. Notably, serum sTim-3 is further upregulated in non-responding patients undergoing anti-PD-1 therapy for NSCLC and anti-PD-1-resistant cholangiocarcinoma patients. Furthermore, sTim-3 overexpression facilitates tumor progression and confers anti-PD-1 resistance in multiple tumor mouse models. Mechanistically, sTim-3 induces terminal T cell exhaustion and attenuates CD8+ T cell response to PD-1 blockade through carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM-1). Moreover, the ADAM10 inhibitor GI254023X, which blocks sTim-3 production, reduces tumor progression in Tim-3 humanized mice and reverses anti-PD-1 resistance in human tumor-infiltrating lymphocytes (TILs). Overall, human sTim-3 holds great predictive and therapeutic potential in onco-immunotherapy.
Keywords: ADAM10; CEACAM-1; HCC; ICC; T cell exhaustion; anti-PD-1 therapy; hepatocellular carcinoma; intrahepatic cholangiocarcinoma; lung cancer; resistance to PD-1 blockade; sTim-3.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests C.L., C.M., C.C., J.P., S.M., and X.L. are inventors on the China patent (ZL202111222514.8) “Application of soluble form of Tim-3 in resistance to immune checkpoint blockade therapy” that has claims directed to sTim-3 application in immunotherapy.
Figures





References
-
- Wherry E.J. T cell exhaustion. Nat. Immunol. 2011;12:492–499. - PubMed
-
- Roncella S., Laurent S., Fontana V., Ferro P., Franceschini M.C., Salvi S., Varesano S., Boccardo S., Vigani A., Morabito A., et al. CTLA-4 in mesothelioma patients: tissue expression, body fluid levels and possible relevance as a prognostic factor. Cancer Immunol. Immunother. 2016;65:909–917. doi: 10.1007/s00262-016-1844-3. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

