Theranostic GPA33-Pretargeted Radioimmunotherapy of Human Colorectal Carcinoma with a Bivalent 177Lu-Labeled Radiohapten
- PMID: 39168519
- PMCID: PMC11448610
- DOI: 10.2967/jnumed.124.267685
Theranostic GPA33-Pretargeted Radioimmunotherapy of Human Colorectal Carcinoma with a Bivalent 177Lu-Labeled Radiohapten
Abstract
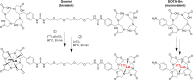
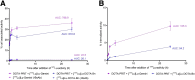
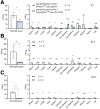
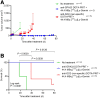
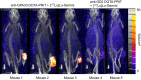
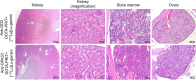
Radiolabeled small-molecule DOTA-haptens can be combined with antitumor/anti-DOTA bispecific antibodies (BsAbs) for pretargeted radioimmunotherapy (PRIT). For optimized delivery of the theranostic γ- and β-emitting isotope 177Lu with DOTA-based PRIT (DOTA-PRIT), bivalent Gemini (DOTA-Bn-thiourea-PEG4-thiourea-Bn-DOTA, aka (3,6,9,12-tetraoxatetradecane-1,14-diyl)bis(DOTA-benzyl thiourea)) was developed. Methods: Gemini was synthesized by linking 2 S-2-(4-isothiocyanatobenzyl)-DOTA molecules together via a 1,14-diamino-PEG4 linker. [177Lu]Lu-Gemini was prepared with no-carrier-added 177LuCl3 to a molar-specific activity of 123 GBq/μmol and radiochemical purity of more than 99%. The specificity of BsAb-177Lu-Gemini was verified in vitro. Subsequently, we evaluated biodistribution and whole-body clearance for [177Lu]Lu-Gemini and, for comparison, our gold-standard monovalent [177Lu]Lu-S-2-(4-aminobenzyl)-DOTA ([177Lu]Lu-DOTA-Bn) in naïve (tumor-free) athymic nude mice. For our proof-of-concept system, a 3-step pretargeting approach was performed with an established DOTA-PRIT regimen (anti-GPA33/anti-DOTA IgG-scFv BsAb, a clearing agent, and [177Lu]Lu-Gemini) in mouse models. Results: Initial in vivo studies showed that [177Lu]Lu-Gemini behaved similarly to [177Lu]Lu-DOTA-Bn, with almost identical blood and whole-body clearance kinetics, as well as biodistribution and mouse kidney dosimetry. Pretargeting [177Lu]Lu-Gemini to GPA33-expressing SW1222 human colorectal xenografts was highly effective, leading to absorbed doses of [177Lu]Lu-Gemini for blood, tumor, liver, spleen, and kidneys of 3.99, 455, 6.93, 5.36, and 14.0 cGy/MBq, respectively. Tumor-to-normal tissue absorbed-dose ratios (i.e., therapeutic indices [TIs]) for the blood and kidneys were 114 and 33, respectively. In addition, we demonstrate that the use of bivalent [177Lu]Lu-Gemini in DOTA-PRIT leads to improved TIs and augmented [177Lu]Lu-Gemini tumor uptake and retention in comparison to monovalent [177Lu]Lu-DOTA-Bn. Finally, we established efficacy in SW1222 tumor-bearing mice, demonstrating that a single injection of anti-GPA33 DOTA-PRIT with 44 MBq (1.2 mCi) of [177Lu]Lu-Gemini (estimated tumor-absorbed dose, 200 Gy) induced complete responses in 5 of 5 animals and a histologic cure in 2 of 5 (40%) animals. Moreover, a significant increase in survival compared with nontreated controls was noted (maximum tolerated dose not reached). Conclusion: We have developed a bivalent DOTA-radiohapten, [177Lu]Lu-Gemini, that showed improved radiopharmacology for DOTA-PRIT application. The use of bivalent [177Lu]Lu-Gemini in DOTA-PRIT, as opposed to monovalent [177Lu]Lu-DOTA-Bn, allows curative treatments with considerably less administered 177Lu activity while still achieving high TIs for both the blood (>100) and the kidneys (>30).
Keywords: 177Lu; GPA33; colorectal cancer; multivalent; pretargeted radioimmunotherapy.
© 2024 by the Society of Nuclear Medicine and Molecular Imaging.
Figures

References
-
- Cheal SM, Chung SK, Vaughn BA, Cheung N-KV, Larson SM. Pretargeting: a path forward for radioimmunotherapy. J Nucl Med. 2022;63:1302–1315. - PubMed
-
- Sgouros G, Dewaraja YK, Escorcia F, et al. . Tumor response to radiopharmaceutical therapies: the knowns and the unknowns. J Nucl Med. 2021;62:12S–22S. - PubMed
-
- Wahl RL, Sgouros G, Iravani A, et al. . Normal-tissue tolerance to radiopharmaceutical therapies, the knowns and the unknowns. J Nucl Med. 2021;62:23S–35S. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
