GAP43 Located on Corticostriatal Terminals Restrains Novelty-Induced Hyperactivity in Mice
- PMID: 39168654
- PMCID: PMC11426381
- DOI: 10.1523/JNEUROSCI.0701-24.2024
GAP43 Located on Corticostriatal Terminals Restrains Novelty-Induced Hyperactivity in Mice
Abstract
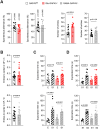
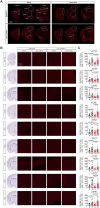
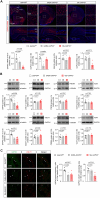
Growth-associated protein of 43 kDa (GAP43) is a key cytoskeleton-associated component of the presynaptic terminal that facilitates neuroplasticity. Downregulation of GAP43 expression has been associated to various psychiatric conditions in humans and evokes hippocampus-dependent memory impairments in mice. Despite the extensive studies conducted on hippocampal GAP43 in past decades, however, very little is known about its roles in modulating the excitatory versus inhibitory balance in other brain regions. We recently generated conditional knock-out mice in which the Gap43 gene was selectively inactivated in either telencephalic glutamatergic neurons (Gap43fl/fl ;Nex1Cre mice, hereafter Glu-GAP43-/- mice) or forebrain GABAergic neurons (Gap43fl/fl ;Dlx5/6Cre mice, hereafter GABA-GAP43-/- mice). Here, we show that Glu-GAP43-/- but not GABA-GAP43-/- mice of either sex show a striking hyperactive phenotype when exposed to a novel environment. This behavioral alteration of Glu-GAP43-/- mice was linked to a selective activation of dorsal-striatum neurons, as well as to an enhanced corticostriatal glutamatergic transmission and an abrogation of corticostriatal endocannabinoid-mediated long-term depression. In line with these observations, GAP43 was abundantly expressed in corticostriatal glutamatergic terminals of wild-type mice. The novelty-induced hyperactive phenotype of Glu-GAP43-/- mice was abrogated by chemogenetically inhibiting corticostriatal afferences with a Gi-coupled "designer receptor exclusively activated by designer drugs" (DREADDs), thus further supporting that novelty-induced activity is controlled by GAP43 at corticostriatal excitatory projections. Taken together, these findings show an unprecedented regulatory role of GAP43 in the corticostriatal circuitry and provide a new mouse model with a delimited neuronal-circuit alteration for studying novelty-induced hyperactivity, a phenotypic shortfall that occurs in diverse psychiatric diseases.
Keywords: GAP43; cannabinoid; corticostriatal circuitry; glutamatergic transmission; long-term depression; motor activity.
Copyright © 2024 Maroto et al.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
