In-depth analysis of transcriptomes in ovarian cortical follicles from children and adults reveals interfollicular heterogeneity
- PMID: 39168975
- PMCID: PMC11339373
- DOI: 10.1038/s41467-024-51185-0
In-depth analysis of transcriptomes in ovarian cortical follicles from children and adults reveals interfollicular heterogeneity
Abstract
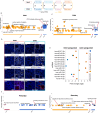
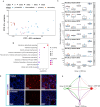
The ovarian cortical reserve of follicles is vital for fertility. Some medical treatments are toxic to follicles, leading to premature ovarian insufficiency. Ovarian tissue cryopreservation is an established method to preserve fertility in adults and even applied in prepuberty despite unproven efficacy. Here, we analyze transcriptomes of 120 cortical follicles from children and adults for detailed comparison. We discover heterogeneity with two main types of follicles in both age groups: one with expected oocyte-granulosa profiles and another with predicted role in signaling. Transcriptional changes during growth to the secondary stage are similar overall in children and adults, but variations related to extracellular matrix, theca cells, and miRNA profiles are found. Notably, cyclophosphamide dose correlates with interferon signaling in child follicles. Additionally, morphology alone is insufficient for follicle categorization suggesting a need for additional markers. Marker genes for early follicle activation are determined. These findings will help refine follicular classification and fertility preservation techniques across critical ages.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
MeSH terms
Substances
Grants and funding
- EU952516/EC | Horizon 2020 Framework Programme (EU Framework Programme for Research and Innovation H2020)
- 825100/EC | Horizon 2020 Framework Programme (EU Framework Programme for Research and Innovation H2020)
- EU952516/EC | Horizon 2020 Framework Programme (EU Framework Programme for Research and Innovation H2020)
- 825100/EC | Horizon 2020 Framework Programme (EU Framework Programme for Research and Innovation H2020)
- PRG1076/Eesti Teadusagentuur (Estonian Research Council)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

