Mechanism and regulation of cargo entry into the Commander endosomal recycling pathway
- PMID: 39168982
- PMCID: PMC11339278
- DOI: 10.1038/s41467-024-50971-0
Mechanism and regulation of cargo entry into the Commander endosomal recycling pathway
Abstract
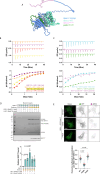
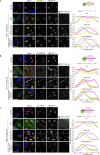
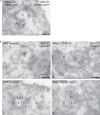
Commander is a multiprotein complex that orchestrates endosomal recycling of integral cargo proteins and is essential for normal development. While the structure of this complex has recently been described, how cargo proteins are selected for Commander-mediated recycling remains unclear. Here we identify the mechanism through which the unstructured carboxy-terminal tail of the cargo adaptor sorting nexin-17 (SNX17) directly binds to the Retriever sub-complex of Commander. SNX17 adopts an autoinhibited conformation where its carboxy-terminal tail occupies the cargo binding groove. Competitive cargo binding overcomes this autoinhibition, promoting SNX17 endosomal residency and the release of the tail for Retriever association. Furthermore, our study establishes the central importance of SNX17-Retriever association in the handover of integrin and lipoprotein receptor cargoes into pre-existing endosomal retrieval sub-domains. In describing the principal mechanism of cargo entry into the Commander recycling pathway we provide key insight into the function and regulation of this evolutionary conserved sorting pathway.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests
Figures





References
-
- Weeratunga, S., Paul, B. & Collins, B. M. Recognising the signals for endosomal trafficking. Curr. Opin. Cell Biol.65, 17–27 (2020). - PubMed
-
- Cullen, P. J. & Steinberg, F. To degrade or not to degrade: mechanisms and significance of endocytic recycling. Nat. Rev. Mol. Cell Biol.19, 679–696 (2018). - PubMed
-
- McNally, K. E. & Cullen, P. J. Endosomal retrieval of cargo: retromer is not alone. Trends Cell Biol.28, 807–822 (2018). - PubMed
-
- Chen, K. E., Healy, M. D. & Collins, B. M. Towards a molecular understanding of endosomal trafficking by retromer and retriever. Traffic20, 465–478 (2019). - PubMed
MeSH terms
Substances
Grants and funding
- 184.034.014/Nederlandse Organisatie voor Wetenschappelijk Onderzoek (Netherlands Organisation for Scientific Research)
- MR/L007363/1/MRC_/Medical Research Council/United Kingdom
- APP1156493/Department of Health | National Health and Medical Research Council (NHMRC)
- APP1136021/Department of Health | National Health and Medical Research Council (NHMRC)
- WT_/Wellcome Trust/United Kingdom
- MR/P018807/1/RCUK | Medical Research Council (MRC)
- 220260/Z/20/Z/Wellcome Trust (Wellcome)
- 953489/EC | EU Framework Programme for Research and Innovation H2020 | H2020 Priority Excellent Science | H2020 European Research Council (H2020 Excellent Science - European Research Council)
- RSRP/R1/211004/Royal Society
LinkOut - more resources
Full Text Sources

