Immune killer cells treatment for previously treated stage IV NSCLC patients
- PMID: 39169058
- PMCID: PMC11339402
- DOI: 10.1038/s41598-024-69587-x
Immune killer cells treatment for previously treated stage IV NSCLC patients
Abstract
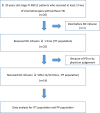
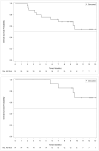
The 5-year survival is poor for stage IV non-small cell lung cancer (NSCLC). Recently, cell immunotherapy has emerged as a new treatment strategy. This study aimed to evaluate the efficacy and safety of Immune killer cells (IKC) in patients with stage IV NSCLC after the failure of prior chemotherapy. This study enrolled 26 patients with stage IV NSCLC who failed at least two lines of chemotherapy with or without targeted therapy. The IKC was given alone weekly for 24 weeks. The primary endpoint was progression-free survival (PFS). Secondary outcomes included overall survival (OS), pain intensity, quality of life (QOL), and safety. The median PFS for the intent-to-treat (ITT) population (i.e., all enrolled patients) was 3.8 month. In the per-protocol (PP) population (i.e., patients receiving > 12 IKC infusions), the median PFS was 5.6 months. Moreover, the ITT population showed a 1-year survival rate of 60.0%, while that for the PP population was 85.7%. Only 7 out of 200 AEs (3.5%) were related to the IKC infusion, and they were all rated as grade 1 in severity. The IKC infusion was well tolerated. This novel immunotherapy prolonged the PFS and improved the survival compared with historical data. It might be a potential treatment strategy for stage IV NSCLC patient who failed prior chemotherapy.ClinicalTrials.gov identifier: NCT03499834.
Keywords: Cell immunotherapy; Immune killer cells; Non-small cell lung cancer; Overall survival; Safety.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Allemani, C. et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): Analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet391, 1023–1075. 10.1016/S0140-6736(17)33326-3 (2018). 10.1016/S0140-6736(17)33326-3 - DOI - PMC - PubMed
-
- Luo, Y. H. et al. 5-year overall survival in patients with lung cancer eligible or ineligible for screening according to US Preventive Services Task Force criteria: A prospective, observational cohort study. Lancet Oncol.20, 1098–1108. 10.1016/S1470-2045(19)30329-8 (2019). 10.1016/S1470-2045(19)30329-8 - DOI - PMC - PubMed
-
- Tseng, Y. H. et al. Efficacy of chemotherapy in epidermal growth factor receptor (EGFR) mutated metastatic pulmonary adenocarcinoma patients who had acquired resistance to first-line EGFR tyrosine kinase inhibitor (TKI). J. Chemother.28, 50–58. 10.1179/1973947815Y.0000000027 (2016). 10.1179/1973947815Y.0000000027 - DOI - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

