Opportunities for use of neuroimaging in de-risking drug development and improving clinical outcomes in psychiatry: an industry perspective
- PMID: 39169213
- PMCID: PMC11526012
- DOI: 10.1038/s41386-024-01970-8
Opportunities for use of neuroimaging in de-risking drug development and improving clinical outcomes in psychiatry: an industry perspective
Erratum in
-
Correction: Neuropsychopharmacology Volume 50 Issue 1.Neuropsychopharmacology. 2025 May;50(6):1019-1020. doi: 10.1038/s41386-025-02087-2. Neuropsychopharmacology. 2025. PMID: 40108440 Free PMC article. No abstract available.
Abstract
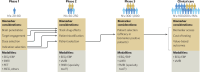
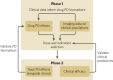
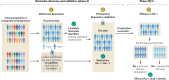
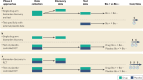
Neuroimaging, across positron emission tomography (PET), electroencephalography (EEG), and magnetic resonance imaging (MRI), has been a mainstay of clinical neuroscience research for decades, yet has penetrated little into psychiatric drug development beyond often underpowered phase 1 studies, or into clinical care. Simultaneously, there is a pressing need to improve the probability of success in drug development, increase mechanistic diversity, and enhance clinical efficacy. These goals can be achieved by leveraging neuroimaging in a precision psychiatry framework, wherein effects of drugs on the brain are measured early in clinical development to understand dosing and indication, and then in later-stage trials to identify likely drug responders and enrich clinical trials, ultimately improving clinical outcomes. Here we examine the key variables important for success in using neuroimaging for precision psychiatry from the lens of biotechnology and pharmaceutical companies developing and deploying new drugs in psychiatry. We argue that there are clear paths for incorporating different neuroimaging modalities to de-risk subsequent development phases in the near to intermediate term, culminating in use of select neuroimaging modalities in clinical care for prescription of new precision drugs. Better outcomes through neuroimaging biomarkers, however, require a wholesale commitment to a precision psychiatry approach and will necessitate a cultural shift to align biopharma and clinical care in psychiatry to a precision orientation already routine in other areas of medicine.
© 2024. The Author(s).
Conflict of interest statement
All authors receive salary and equity from Alto Neuroscience, Inc. AE holds equity from Akili Interactive and AJS holds equity from Johnson and Johnson.
Figures
References
-
- Blokland A, Heckman P, Vanmierlo T, Schreiber R, Paes D, Prickaerts J. Phosphodiesterase Type 4 Inhibition in CNS Diseases. Trends Pharmacol Sci. 2019;40:971–85. - PubMed
-
- Bolger GB. The PDE4 cAMP-Specific Phosphodiesterases: Targets for Drugs with Antidepressant and Memory-Enhancing Action. In: Zhang H-T, Xu Y, O’Donnell JM, editors. Phosphodiesterases: CNS Functions and Diseases, vol. 17, pp.63–102. Cham: Springer International Publishing; 2017. - PubMed
-
- Blokland A, Van Duinen MA, Sambeth A, Heckman P, Tsai M, Lahu G, et al. Acute treatment with the PDE4 inhibitor roflumilast improves verbal word memory in healthy old individuals: a double-blind placebo-controlled study. Neurobiol Aging. 2019;77:37–43. - PubMed
-
- Gilleen J, Nottage J, Yakub F, Kerins S, Valdearenas L, Uz T, et al. The effects of roflumilast, a phosphodiesterase type-4 inhibitor, on EEG biomarkers in schizophrenia: A randomised controlled trial. J Psychopharmacol. 2021;35:15–22. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

