CLDN6 inhibits breast cancer growth and metastasis through SREBP1-mediated RAS palmitoylation
- PMID: 39169280
- PMCID: PMC11337767
- DOI: 10.1186/s11658-024-00629-y
CLDN6 inhibits breast cancer growth and metastasis through SREBP1-mediated RAS palmitoylation
Abstract
Background: Breast cancer (BC) ranks as the third most fatal malignant tumor worldwide, with a strong reliance on fatty acid metabolism. CLDN6, a candidate BC suppressor gene, was previously identified as a regulator of fatty acid biosynthesis; however, the underlying mechanism remains elusive. In this research, we aim to clarify the specific mechanism through which CLDN6 modulates fatty acid anabolism and its impact on BC growth and metastasis.
Methods: Cell function assays, tumor xenograft mouse models, and lung metastasis mouse models were conducted to evaluate BC growth and metastasis. Human palmitic acid assay, triglyceride assay, Nile red staining, and oil red O staining were employed to investigate fatty acid anabolism. Reverse transcription polymerase chain reaction (RT-PCR), western blot, immunohistochemistry (IHC) assay, nuclear fractionation, immunofluorescence (IF), immunoprecipitation and acyl-biotin exchange (IP-ABE), chromatin immunoprecipitation (ChIP), dual luciferase reporter assay, and co-immunoprecipitation (Co-IP) were applied to elucidate the underlying molecular mechanism. Moreover, tissue microarrays of BC were analyzed to explore the clinical implications.
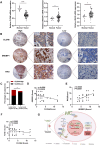
Results: We identified that CLDN6 inhibited BC growth and metastasis by impeding RAS palmitoylation both in vitro and in vivo. We proposed a unique theory suggesting that CLDN6 suppressed RAS palmitoylation through SREBP1-modulated de novo palmitic acid synthesis. Mechanistically, CLDN6 interacted with MAGI2 to prevent KLF5 from entering the nucleus, thereby restraining SREBF1 transcription. The downregulation of SREBP1 reduced de novo palmitic acid synthesis, hindering RAS palmitoylation and subsequent endosomal sorting complex required for transport (ESCRT)-mediated plasma membrane localization required for RAS oncogenic activation. Besides, targeting inhibition of RAS palmitoylation synergized with CLDN6 to repress BC progression.
Conclusions: Our findings provide compelling evidence that CLDN6 suppresses the palmitic acid-induced RAS palmitoylation through the MAGI2/KLF5/SREBP1 axis, thereby impeding BC malignant progression. These results propose a new insight that monitoring CLDN6 expression alongside targeting inhibition of palmitic acid-mediated palmitoylation could be a viable strategy for treating oncogenic RAS-driven BC.
Keywords: Breast cancer; CLDN6; Fatty acid; Palmitoylation; RAS; SREBP1.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures







References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clin. 2021;71(3):209–49. - PubMed
-
- Loke SY, Lee ASG. The future of blood-based biomarkers for the early detection of breast cancer. Eur J Cancer. 2018;92:54–68. - PubMed
-
- Rajput S, Sharma PK, Malviya R. Biomarkers and treatment strategies for breast cancer recurrence. Curr Drug Targets. 2023;24(15):1209–20. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

