A systematic review and meta-analysis of randomized trials of substituting soymilk for cow's milk and intermediate cardiometabolic outcomes: understanding the impact of dairy alternatives in the transition to plant-based diets on cardiometabolic health
- PMID: 39169353
- PMCID: PMC11340166
- DOI: 10.1186/s12916-024-03524-7
A systematic review and meta-analysis of randomized trials of substituting soymilk for cow's milk and intermediate cardiometabolic outcomes: understanding the impact of dairy alternatives in the transition to plant-based diets on cardiometabolic health
Abstract
Background: Dietary guidelines recommend a shift to plant-based diets. Fortified soymilk, a prototypical plant protein food used in the transition to plant-based diets, usually contains added sugars to match the sweetness of cow's milk and is classified as an ultra-processed food. Whether soymilk can replace minimally processed cow's milk without the adverse cardiometabolic effects attributed to added sugars and ultra-processed foods remains unclear. We conducted a systematic review and meta-analysis of randomized controlled trials, to assess the effect of substituting soymilk for cow's milk and its modification by added sugars (sweetened versus unsweetened) on intermediate cardiometabolic outcomes.
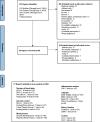
Methods: MEDLINE, Embase, and the Cochrane Central Register of Controlled Trials were searched (through June 2024) for randomized controlled trials of ≥ 3 weeks in adults. Outcomes included established markers of blood lipids, glycemic control, blood pressure, inflammation, adiposity, renal disease, uric acid, and non-alcoholic fatty liver disease. Two independent reviewers extracted data and assessed risk of bias. The certainty of evidence was assessed using GRADE (Grading of Recommendations, Assessment, Development, and Evaluation). A sub-study of lactose versus sucrose outside of a dairy-like matrix was conducted to explore the role of sweetened soymilk which followed the same methodology.
Results: Eligibility criteria were met by 17 trials (n = 504 adults with a range of health statuses), assessing the effect of a median daily dose of 500 mL of soymilk (22 g soy protein and 17.2 g or 6.9 g/250 mL added sugars) in substitution for 500 mL of cow's milk (24 g milk protein and 24 g or 12 g/250 mL total sugars as lactose) on 19 intermediate outcomes. The substitution of soymilk for cow's milk resulted in moderate reductions in non-HDL-C (mean difference, - 0.26 mmol/L [95% confidence interval, - 0.43 to - 0.10]), systolic blood pressure (- 8.00 mmHg [- 14.89 to - 1.11]), and diastolic blood pressure (- 4.74 mmHg [- 9.17 to - 0.31]); small important reductions in LDL-C (- 0.19 mmol/L [- 0.29 to - 0.09]) and c-reactive protein (CRP) (- 0.82 mg/L [- 1.26 to - 0.37]); and trivial increases in HDL-C (0.05 mmol/L [0.00 to 0.09]). No other outcomes showed differences. There was no meaningful effect modification by added sugars across outcomes. The certainty of evidence was high for LDL-C and non-HDL-C; moderate for systolic blood pressure, diastolic blood pressure, CRP, and HDL-C; and generally moderate-to-low for all other outcomes. We could not conduct the sub-study of the effect of lactose versus added sugars, as no eligible trials could be identified.
Conclusions: Current evidence provides a good indication that replacing cow's milk with soymilk (including sweetened soymilk) does not adversely affect established cardiometabolic risk factors and may result in advantages for blood lipids, blood pressure, and inflammation in adults with a mix of health statuses. The classification of plant-based dairy alternatives such as soymilk as ultra-processed may be misleading as it relates to their cardiometabolic effects and may need to be reconsidered in the transition to plant-based diets.
Trial registration: ClinicalTrials.gov identifier, NCT05637866.
Keywords: Cardiovascular disease; Meta-analysis; Randomized controlled feeding trials; Soy protein; Soymilk; Systematic review.
© 2024. The Author(s).
Conflict of interest statement
TAK reports receiving grants from Institute for the Advancement of Food and Nutrition Sciences (IAFNS, formerly ILSI North America) and National Honey Board (USDA Checkoff program). He has received honorariums from Advancement of Food and Nutrition Sciences (IAFNS), the International Food Information Council (IFIC), the Calorie Control Council (CCC), the International Sweeteners Association (ISA), and AmCham Dubai. He has received funding from the Toronto 3D Knowledge Synthesis and Clinical Trials foundation. LC has received research support from the Canadian Institutes of health Research (CIHR), Protein Industries Canada (a Government of Canada Global Innovation Clusters), The United Soybean Board (USDA soy “Checkoff” program), and the Alberta Pulse Growers Association. AZ is a part-time research associate at INQUIS Clinical Research, Ltd., a contract research organization. She has received consulting fees from Glycemic Index Foundation Inc. SA-C has received an honorarium from the International Food Information Council (IFIC) for a talk on artificial sweeteners, the gut microbiome, and the risk for diabetes. MM was employed by the Soy Nutrition Institute Global, an organization that receives funding from the United Soybean Board (USB) and from members involved in the soy industry. RPB has received industrial grants, including those matched by the Canadian government, and/or travel support or consulting fees largely related to work on brain fatty acid metabolism or nutrition from Arctic Nutrition, Bunge Ltd., Dairy Farmers of Canada, DSM, Fonterra Inc, Mead Johnson, Natures Crops International, Nestec Inc. Pharmavite, Sancero Inc., and Spore Wellness Inc. Moreover, Dr. Bazinet is on the executive of the International Society for the Study of Fatty Acids and Lipids and held a meeting on behalf of Fatty Acids and Cell Signaling, both of which rely on corporate sponsorship. Dr. Bazinet has given expert testimony in relation to supplements and the brain. DJAJ has received research grants from Saskatchewan & Alberta Pulse Growers Associations, the Agricultural Bioproducts Innovation Program through the Pulse Research Network, the Advanced Foods and Material Network, Loblaw Companies Ltd., Unilever Canada and Netherlands, Barilla, the Almond Board of California, Agriculture and Agri-food Canada, Pulse Canada, Kellogg’s Company, Canada, Quaker Oats, Canada, Procter & Gamble Technical Centre Ltd., Bayer Consumer Care, Springfield, NJ, Pepsi/Quaker, International Nut & Dried Fruit Council (INC), Soy Foods Association of North America, the Coca-Cola Company (investigator initiated, unrestricted grant), Solae, Haine Celestial, the Sanitarium Company, Orafti, the International Tree Nut Council Nutrition Research and Education Foundation, the Peanut Institute, Soy Nutrition Institute (SNI), the Canola and Flax Councils of Canada, the Calorie Control Council, the Canadian Institutes of Health Research (CIHR), the Canada Foundation for Innovation (CFI), and the Ontario Research Fund (ORF). He has received in-kind supplies for trials as a research support from the Almond board of California, Walnut Council of California, the Peanut Institute, Barilla, Unilever, Unico, Primo, Loblaw Companies, Quaker (Pepsico), Pristine Gourmet, Bunge Limited, Kellogg Canada, and WhiteWave Foods. He has been on the speaker’s panel, served on the scientific advisory board and/or received travel support and/or honoraria from Lawson Centre Nutrition Digital Series, Nutritional Fundamentals for Health (NFH)-Nutramedica, Saint Barnabas Medical Center, The University of Chicago, 2020 China Glycemic Index (GI) International Conference, Atlantic Pain Conference, Academy of Life Long Learning, the Almond Board of California, Canadian Agriculture Policy Institute, Loblaw Companies Ltd, the Griffin Hospital (for the development of the NuVal scoring system), the Coca-Cola Company, Epicure, Danone, Diet Quality Photo Navigation (DQPN), Better Therapeutics (FareWell), Verywell, True Health Initiative (THI), Heali AI Corp, Institute of Food Technologists (IFT), Soy Nutrition Institute (SNI), Herbalife Nutrition Institute (HNI), Saskatchewan & Alberta Pulse Growers Associations, Sanitarium Company, Orafti, the International Tree Nut Council Nutrition Research and Education Foundation, the Peanut Institute, Herbalife International, Pacific Health Laboratories, Barilla, Metagenics, Bayer Consumer Care, Unilever Canada and Netherlands, Solae, Kellogg, Quaker Oats, Procter & Gamble, Abbott Laboratories, Dean Foods, the California Strawberry Commission, Haine Celestial, PepsiCo, the Alpro Foundation, Pioneer Hi-Bred International, DuPont Nutrition and Health, Spherix Consulting and WhiteWave Foods, the Advanced Foods and Material Network, the Canola and Flax Councils of Canada, Agri-Culture and Agri-Food Canada, the Canadian Agri-Food Policy Institute, Pulse Canada, the Soy Foods Association of North America, the Nutrition Foundation of Italy (NFI), Nutra-Source Diagnostics, the McDougall Program, the Toronto Knowledge Translation Group (St. Michael’s Hospital), the Canadian College of Naturopathic Medicine, The Hospital for Sick Children, the Canadian Nutrition Society (CNS), the American Society of Nutrition (ASN), Arizona State University, Paolo Sorbini Foundation, and the Institute of Nutrition, Metabolism and Diabetes. He received an honorarium from the United States Department of Agriculture to present the 2013 W.O. Atwater Memorial Lecture. He received the 2013 Award for Excellence in Research from the International Nut and Dried Fruit Council. He received funding and travel support from the Canadian Society of Endocrinology and Metabolism to produce mini cases for the Canadian Diabetes Association (CDA). He is a member of the International Carbohydrate Quality Consortium (ICQC). His wife, Alexandra L Jenkins, is a director and partner of INQUIS Clinical Research for the Food Industry, his 2 daughters, Wendy Jenkins and Amy Jenkins, have published a vegetarian book that promotes the use of the foods described here, The Portfolio Diet for Cardiovascular Risk Reduction (Academic Press/Elsevier 2020 ISBN:978–0-12–810510-8), and his sister, Caroline Brydson, received funding through a grant from the St. Michael’s Hospital Foundation to develop a cookbook for one of his studies. He is also a vegan. CWCK has received grants or research support from the Advanced Food Materials Network, Agriculture and Agri-Foods Canada (AAFC), Almond Board of California, Barilla, Canadian Institutes of Health Research (CIHR), Canola Council of Canada, International Nut and Dried Fruit Council, International Tree Nut Council Research and Education Foundation, Loblaw Brands Ltd, the Peanut Institute, Pulse Canada, and Unilever. He has received in-kind research support from the Almond Board of California, Barilla, California Walnut Commission, Kellogg Canada, Loblaw Companies, Nutrartis, Quaker (PepsiCo), the Peanut Institute, Primo, Unico, Unilever, and WhiteWave Foods/Danone. He has received travel support and/or honoraria from the Barilla, California Walnut Commission, Canola Council of Canada, General Mills, International Nut and Dried Fruit Council, International Pasta Organization, Lantmannen, Loblaw Brands Ltd, Nutrition Foundation of Italy, Oldways Preservation Trust, Paramount Farms, the Peanut Institute, Pulse Canada, Sun-Maid, Tate & Lyle, Unilever, and White Wave Foods/Danone. He has served on the scientific advisory board for the International Tree Nut Council, International Pasta Organization, McCormick Science Institute, and Oldways Preservation Trust. He is a founding member of the International Carbohydrate Quality Consortium (ICQC), Executive Board Member of the Diabetes and Nutrition Study Group (DNSG) of the European Association for the Study of Diabetes (EASD), is on the Clinical Practice Guidelines Expert Committee for Nutrition Therapy of the EASD, and is a Director of the Toronto 3D Knowledge Synthesis and Clinical Trials foundation. JLS has received research support from the Canadian Foundation for Innovation, Ontario Research Fund, Province of Ontario Ministry of Research and Innovation and Science, Canadian Institutes of health Research (CIHR), Diabetes Canada, American Society for Nutrition (ASN), National Honey Board (U.S. Department of Agriculture [USDA] honey “Checkoff” program), Institute for the Advancement of Food and Nutrition Sciences (IAFNS), Pulse Canada, Quaker Oats Center of Excellence, INC International Nut and Dried Fruit Council Foundation, The United Soybean Board (USDA soy “Checkoff” program), Protein Industries Canada (a Government of Canada Global Innovation Cluster), Almond Board of California, European Fruit Juice Association, The Tate and Lyle Nutritional Research Fund at the University of Toronto, The Glycemic Control and Cardiovascular Disease in Type 2 Diabetes Fund at the University of Toronto (a fund established by the Alberta Pulse Growers), The Plant Protein Fund at the University of Toronto (a fund which has received contributions from IFF among other donors), The Plant Milk Fund at the University of Toronto (a fund established by the Karuna Foundation through Vegan Grants), and The Nutrition Trialists Network Fund at the University of Toronto (a fund established by donations from the Calorie Control Council and Physicians Committee for Responsible Medicine). He has received food donations to support randomized controlled trials from the Almond Board of California, California Walnut Commission, Danone, Nutrartis, Soylent, and Dairy Farmers of Canada. He has received travel support, speaker fees and/or honoraria from Danone, FoodMinds LLC, Nestlé, Abbott, General Mills, Nutrition Communications, International Food Information Council (IFIC), Arab Beverages, International Sweeteners Association, Association Calorie Control Council, and Phynova. He has or has had ad hoc consulting arrangements with Perkins Coie LLP, Tate & Lyle, Ingredion, and Brightseed. He is on the Clinical Practice Guidelines Expert Committees of Diabetes Canada, European Association for the study of Diabetes (EASD), Canadian Cardiovascular Society (CCS), and Obesity Canada/Canadian Association of Bariatric Physicians and Surgeons. He serves as an unpaid member of the Board of Trustees of IAFNS. He is a Director at Large of the Canadian Nutrition Society (CNS), founding member of the International Carbohydrate Quality Consortium (ICQC), Executive Board Member of the Diabetes and Nutrition Study Group (DNSG) of the EASD, and Director of the Toronto 3D Knowledge Synthesis and Clinical Trials foundation. His spouse is an employee of AB InBev. All other authors declare that they have no competing interests.
Figures

References
-
- Dietary guidelines for Americans, 2020–2025. 2020 [9:[Available from: www.dietaryguidelines.gov.
-
- Canada, Health. Canada’s Food Guide. Ottawa; 2019. https://food-guide.canada.ca/en/.
-
- Canada’s food guide Ottawa 2019 [Available from: https://food-guide.canada.ca/en/.
-
- Blomhoff R, Andersen R, Arnesen EK, Christensen JJ, Eneroth H, Erkkola M, Gudanaviciene I, Halldórsson ÞI, Höyer-Lund A, Lemming EW. Nordic nutrition recommendations 2023: integrating environmental aspects. Nordisk Ministerråd; 2023.
-
- García EL, Lesmes IB, Perales AD, Arribas VM, del Puy Portillo Baquedano M, Velasco AMR, Salvo UF, Romero LT, Porcel FBO, Laín SA. Report of the Scientific Committee of the Spanish Agency for Food Safety and Nutrition (AESAN) on sustainable dietary and physical activity recommendations for the Spanish population. Wiley Online Library; 2023. Report No.: 2940–1399.
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

