TALEN-edited allogeneic inducible dual CAR T cells enable effective targeting of solid tumors while mitigating off-tumor toxicity
- PMID: 39169622
- PMCID: PMC11573618
- DOI: 10.1016/j.ymthe.2024.08.018
TALEN-edited allogeneic inducible dual CAR T cells enable effective targeting of solid tumors while mitigating off-tumor toxicity
Abstract
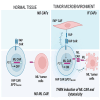
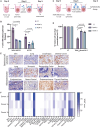
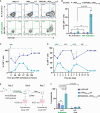
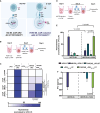
Adoptive cell therapy using chimeric antigen receptor (CAR) T cells has proven to be lifesaving for many cancer patients. However, its therapeutic efficacy has been limited in solid tumors. One key factor for this is cancer-associated fibroblasts (CAFs) that modulate the tumor microenvironment (TME) to inhibit T cell infiltration and induce "T cell dysfunction." Additionally, the sparsity of tumor-specific antigens (TSA) and expression of CAR-directed tumor-associated antigens (TAA) on normal tissues often results in "on-target off-tumor" cytotoxicity, raising safety concerns. Using TALEN-mediated gene editing, we present here an innovative CAR T cell engineering strategy to overcome these challenges. Our allogeneic "Smart CAR T cells" are designed to express a constitutive CAR, targeting FAP+ CAFs in solid tumors. Additionally, a second CAR targeting a TAA such as mesothelin is specifically integrated at a TCR signaling-inducible locus like PDCD1. FAPCAR-mediated CAF targeting induces expression of the mesothelin CAR, establishing an IF/THEN-gated circuit sensitive to dual antigen sensing. Using this approach, we observe enhanced anti-tumor cytotoxicity, while limiting "on-target off-tumor" toxicity. Our study thus demonstrates TALEN-mediated gene editing capabilities for design of allogeneic IF/THEN-gated dual CAR T cells that efficiently target immunotherapy-recalcitrant solid tumors while mitigating potential safety risks, encouraging clinical development of this strategy.
Keywords: CAR T cell; CAR logic gate; TALEN; allogeneic; cell therapy; gene editing; immunotherapy; on-target off-tumor toxicity; solid tumors.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests S.Dharani, H.C., A.J., J.V., P.D., L.P., and S.Das are current employees and equity holders at Cellectis. TALEN is a Cellectis patented technology. J.P.F. is a former Cellectis employee and is a current employee of Bristol Myers Squibb.
Figures

References
-
- Brentjens R.J., Rivière I., Park J.H., Davila M.L., Wang X., Stefanski J., Taylor C., Yeh R., Bartido S., Borquez-Ojeda O., et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. 2011;118:4817–4828. doi: 10.1182/blood-2011-04-348540. - DOI - PMC - PubMed
-
- Westin J.R., Kersten M.J., Salles G., Abramson J.S., Schuster S.J., Locke F.L., Andreadis C. Efficacy and safety of CD19-directed CAR-T cell therapies in patients with relapsed/refractory aggressive b-cell lymphomas: Observations from the JULIET, ZUMA-1, and TRANSCEND trials. Am. J. Hematol. 2021;96:1295–1312. doi: 10.1002/ajh.26301. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

