Hydroboration of Terminal Alkynes Catalyzed by a Mn(I) Alkyl PCP Pincer Complex Following Two Diverging Pathways
- PMID: 39169905
- PMCID: PMC11334104
- DOI: 10.1021/acscatal.4c03805
Hydroboration of Terminal Alkynes Catalyzed by a Mn(I) Alkyl PCP Pincer Complex Following Two Diverging Pathways
Abstract
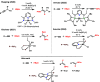
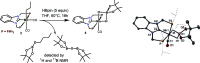
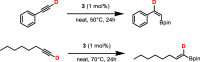
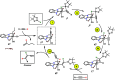
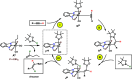
A stereo- and regioselective Mn(I)-catalyzed hydroboration of terminal alkynes with pinacolborane (HBPin) is described. The hydroboration reaction is highly Z-selective in the case of aryl alkynes and E-selective in the case of aliphatic alkynes. The reaction requires no additives or solvents and proceeds with a catalyst loading of 1 mol % at 50-70 °C. The most active precatalyst is the bench-stable alkyl Mn(I) complex cis-[Mn(PCP-iPr)(CO)2(CH2CH2CH3)]. The catalytic process is initiated by the migratory insertion of a CO ligand into the Mn-alkyl bond to yield an acyl intermediate. This species undergoes C-H and B-H bond cleavage of the alkyne (aromatic alkynes) and HBPin (in the case of aliphatic alkynes) forming the active Mn(I) alkynyl and boryl catalysts [Mn(PCP-iPr)(CO)(C≡CR)] and [Mn(PCP-iPr)(CO)(BPin)], respectively. A broad variety of aromatic and aliphatic alkynes was efficiently and selectively borylated. Mechanistic insights are provided based on experimental data and DFT calculations. The functionalized alkenes can be used for further applications in cross-coupling reactions.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Miyaura N.; Yamada K.; Suzuki A. A New Stereospecific Cross-Coupling by the Palladium-Catalyzed Reaction of 1-Alkenylboranes with 1-Alkenyl or 1-Alkynyl Halides. Tetrahedron Lett. 1979, 20, 3437–3440. 10.1016/S0040-4039(01)95429-2. - DOI
- Suzuki A.; Miyaura N. Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds. Chem. Rev. 1995, 95, 2457–2483. 10.1021/cr00039a007. - DOI
- Martin R.; Buchwald S. L. Palladium-Catalyzed Suzuki– Miyaura Cross-Coupling Reactions Employing Dialkylbiaryl Phosphine Ligands. Acc. Chem. Res. 2008, 41, 1461–1473. 10.1021/ar800036s. - DOI - PMC - PubMed
- Hooshmand S. E.; Heidari B.; Sedghi R.; Varma R. S. Recent Advances in the Suzuki–Miyaura Cross-Coupling Reaction Using Efficient Catalysts in Eco-Friendly Media. Green Chem. 2019, 21, 381–405. 10.1039/C8GC02860E. - DOI
-
- Brown H.; Rao B. C. Communications - Hydroboration of Olefins. A Remakably Fast Room-Temperature Addition of Diborane to Olefins. J. Org. Chem. 1957, 22, 1136–1137. 10.1021/jo01360a625. - DOI
- Brown H. C.; Zweifel G. Hydroboration. IX. The Hydroboration of Cyclic and Bicyclic Olefins—Stereochemistry of the Hydroboration Reaction. J. Am. Chem. Soc. 1961, 83, 2544–2551. 10.1021/ja01472a028. - DOI
-
- Vogels C. M.; Westcott S. A. Recent Advances in Organic Synthesis Using Transition Metal-Catalyzed Hydroborations. Curr. Org. Chem. 2005, 9, 687–699. 10.2174/1385272053765060. - DOI
-
- Metal-Catalyzed Hydroboration Reactions. In: Transition Metals for Organic Synthesis: Building Blocks and Fine Chemicals (Eds.: Beller M.; Bolm C.), Wiley-VCH: Weinheim, 1998.
- Contemporary Boron Chemistry (Eds.: Wade M. G.; Marder K.; Hughes A. K.), Royal Society of Chemistry: Cambridge, 2000.
-
-
For selected examples for Rh-catalyzed hydroboration reactions see:
- Kono H.; Ito K.; Nagai Y. Oxidative Addition of 4,4,6-Trimethyl-1,3,2-Dioxaborinane and Benzo[1,3,2]Dioxaborole to Tris(Triphenylphosphine)Halogenorhodium. Chem. Lett. 1975, 4, 1095–1096. 10.1246/cl.1975.1095. - DOI
- Brown J. M.; Hulmes D. I.; Layzell T. P.. Effective Asymmetric Hydroboration Catalysed by a Rhodium Complex of 1-(2-Diphenylphosphino-1-Naphthyl) Isoquinoline. J. Chem. Soc., Chem. Commun. 1993, 1673–1674. 10.1039/c39930001673 - DOI
- Smith J. R.; Collins B. S. L.; Hesse M. J.; Graham M. A.; Myers E. L.; Aggarwal V. K. Enantioselective Rhodium(III)-Catalyzed Markovnikov Hydroboration of Unactivated Terminal Alkenes. J. Am. Chem. Soc. 2017, 139, 9148–9151. 10.1021/jacs.7b05149. - DOI - PMC - PubMed
-
LinkOut - more resources
Full Text Sources
