Diagnostic and prognostic biomarkers in immune checkpoint inhibitor-related encephalitis: a retrospective cohort study
- PMID: 39170102
- PMCID: PMC11338149
- DOI: 10.1016/j.lanepe.2024.101011
Diagnostic and prognostic biomarkers in immune checkpoint inhibitor-related encephalitis: a retrospective cohort study
Abstract
Background: Immune checkpoint inhibitor-related encephalitis (ICI-encephalitis) is not well characterised and diagnostic and prognostic biomarkers are lacking. We aimed to comprehensively characterise ICI-encephalitis and identify diagnostic biomarkers and outcome predictors.
Methods: This retrospective observational study included all patients with ICI-encephalitis studied in the French Reference Centre on Paraneoplastic Neurological Syndromes (PNS) and Autoimmune Encephalitis (2015-2023). ICI encephalitis was considered definite in case of inflammatory findings at paraclinical tests and/or well-characterised neural antibodies. Predictors of immune-related adverse event (irAE) treatment response, defined as a Common Terminology Criteria for Adverse Events v5.0 grade < 3 at any time after therapeutic intervention, were assessed by logistic regression analysis, and predictors of mortality by Cox regression analysis. Neurofilament light chain (NfL) was measured by enzyme-linked immunosorbent assay.
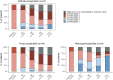
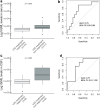
Findings: Sixty-seven patients with definite encephalitis were identified (median age, 69 years; 66% male). A focal syndrome was observed in 43/67 patients (64%; limbic encephalitis, cerebellar ataxia, and/or brainstem encephalitis), while 24/67 (36%) had meningoencephalitis, a non-focal syndrome with altered mental status (22/24 patients, 92%) and pleocytosis (24/24 patients, 100%). Patients with focal encephalitis more frequently had abnormal brain MRI (26/42, 62% versus 8/24, 33%, p = 0.025), PNS-related antibodies (36/43, 84% versus 1/24, 4%, p < 0.001), and neuroendocrine cancers (22/43, 51% versus 1/24, 4%; p < 0.001) than patients with meningoencephalitis. Focal encephalitis patients had a lower rate of irAE treatment response (7/39, 18%) and higher mortality (27/43, 63%) compared to meningoencephalitis patients (12/22, 77% and 5/24, 21%, respectively, p < 0.001 each). PNS-related antibodies were associated with less irAE treatment response, independently of age, sex, and baseline severity (adjusted OR 0.05; 95%CI [0.01; 0.19]; p < 0.001) as well as higher mortality, independently of age and cancer type (adjusted HR 5.07; 95% CI [2.12; 12.12]; p < 0.001). Serum NfL discriminated patients with definite ICI-encephalitis (n = 27) from cancer-matched controls (n = 16; optimal cut-off >273.5 pg/mL, sensitivity 81%, specificity 88%, AUC 0.87, 95% CI [0.76; 0.98]) and irAE treatment responders (n = 10) from non-responders (n = 17, optimal cut-off >645 pg/mL, sensitivity 90%, specificity 65%; AUC 0.75, 95% CI [0.55; 0.94]).
Interpretation: ICI-encephalitis corresponds to a set of clinically-recognisable syndromes. Patients with focal encephalitis, PNS-related antibodies, and/or higher serum NfL have low irAE treatment response rates. Research is needed on the underlying immunopathogenesis to foster therapeutic innovations.
Funding: Agence Nationale de la Recherche.
Keywords: Adverse events; Autoimmune encephalitis; Biomarkers; Cancer; Cancer immunotherapy; Encephalitis; Immune checkpoint inhibitors; Neural antibodies; Neurological immune-related adverse events; Paraneoplastic neurological syndromes; Prognostic factors.
© 2024 The Author(s).
Conflict of interest statement
AF, MVG, BJ, and JH are supported by BETPSY, project as part of the second Investissements d'Avenir programme (ANR-18-RHUS-0012) supported by a public grant overseen by the Agence Nationale de la Recherche (ANR). AF received a grant to perform research abroad by the European Academy of Neurology. MVG is supported by Fundación Martín Escudero (non-profit Spanish foundation) to perform research abroad. JH receives royalties from licensing fees to Athena Diagnostics, Euroimmun, and ravo Diagnostika for a patent for the use of anti-CV2/CRMP5 as diagnostic tests. All other authors declare no competing interests.
Figures



References
-
- Postow M.A., Sidlow R., Hellmann M.D. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378:158–168. - PubMed
-
- Arnaud-Coffin P., Maillet D., Gan H.K., et al. A systematic review of adverse events in randomized trials assessing immune checkpoint inhibitors. Int J Cancer. 2019;145:639–648. - PubMed
-
- Marini A., Bernardini A., Gigli G.L., et al. Neurologic adverse events of immune checkpoint inhibitors: a systematic review. Neurology. 2021;96:754–766. - PubMed
LinkOut - more resources
Full Text Sources

