CXCL12-loaded-hydrogel (CLG): A new device for metastatic circulating tumor cells (CTCs) capturing and characterization
- PMID: 39170328
- PMCID: PMC11336720
- DOI: 10.1016/j.heliyon.2024.e35524
CXCL12-loaded-hydrogel (CLG): A new device for metastatic circulating tumor cells (CTCs) capturing and characterization
Abstract
Background: Circulating Tumor Cells (CTCs) represent a small, heterogeneous population that comprise the minority of cells able to develop metastasis. To trap and characterize CTCs with metastatic attitude, a CXCL12-loaded hyaluronic-gel (CLG) was developed. CXCR4+cells with invasive capability would infiltrate CLG.
Methods: Human colon, renal, lung and ovarian cancer cells (HT29, A498, H460 and OVCAR8 respectively) were seeded on 150 μl Empty Gels (EG) or 300 ng/ml CXCL12 loaded gel (CLG) and allowed to infiltrate for 16 h. Gels were then digested and fixed with 2 % FA-HAse for human cancer cell enumeration or digested with HAse and cancer cells recovered. CLG-recovered cells migrated toward CXCL12 and were tested for colonies/spheres formation. Moreover, CXCR4, E-Cadherin and Vimentin expression was assessed through flow cytometry and RT-PCR. The clinical trial "TRAP4MET" recruited 48 metastatic/advanced cancer patients (8 OC, 8 LC, 8 GBM, 8 EC, 8 RCC and 8 EC). 10 cc whole blood were devoted to PBMCs extraction (7 cc) and ScreenCell™ filters (3 cc) CTCs evaluation. Ficoll-isolated patient's PBMCs were seeded over CLG and allowed to infiltrate for 16 h; gels were digested and fixed with 2 % FA-HAse, cells stained and DAPI+/CD45-/pan-CK + cells enumerated as CTCs.
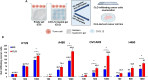
Results: Human cancer cells infiltrate CLG more efficiently than EG (CLG/EG ratio 1.25 for HT29/1.58 for A498/1.71 for H460 and 2.83 for OVCAR8). CLG-recovered HT29 cells display hybrid-mesenchymal features [low E-cadherin (40 %) and high vimentin (235 %) as compared to HT29], CXCR4 two-fold higher than HT29, efficiently migrate toward CXCL12 (two-fold higher than HT29) and developed higher number of colonies (171 ± 21 for HT29-CLG vs 131 ± 8 colonies for HT29)/larger spheres (spheroid area: 26561 ± 6142 μm2 for HT29-CLG vs 20297 ± 7238 for HT29). In TRAP4MET clinical trial, CLG-CTCs were isolated in 8/8 patients with OC, 6/8 with LC, 6/8 with CRC, 8/8 with EC, 8/8 with RCC cancer and 5/8 with GBM. Interestingly, in OC, LC and GBM, CLG isolated higher number of CTCs as compared to the conventional ScreenCell™ (CLG/SC ratio = 1.88 for OC, 2.47 for LC and 11.89 for GBM). Bland and Altman blot analysis and Passing and Bablok regression analysis showed concordance between the methodological approaches but indicate that SC and CLG are not superimposable suggesting that the two systems select cells with different features.
Conclusion: CLG might represent a new and easy tool to isolate invasive CTCs in multiple cancers such as OC, LC and GBM at today orphan of reliable methods to consistently detect CTCs.
Keywords: Artificial niche; CXCL12/CXCR4; Cancer trap; Metastatic CTCs.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- Peinado H., Zhang H., Matei I.R., Costa-Silva B., Hoshino A., Rodrigues G., Psaila B., Kaplan R.N., Bromberg J.F., Kang Y., Bissell M.J., Cox T.R., Giaccia A.J., Erler J.T., Hiratsuka S., Ghajar C.M., Lyden D. Pre-metastatic niches: organ-specific homes for metastases. Nat. Rev. Cancer. 2017;17:302–317. doi: 10.1038/nrc.2017.6. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

