Human Aortic Stenotic Valve-Derived Extracellular Vesicles Induce Endothelial Dysfunction and Thrombogenicity Through AT1R/NADPH Oxidases/SGLT2 Pro-Oxidant Pathway
- PMID: 39170957
- PMCID: PMC11334416
- DOI: 10.1016/j.jacbts.2024.02.012
Human Aortic Stenotic Valve-Derived Extracellular Vesicles Induce Endothelial Dysfunction and Thrombogenicity Through AT1R/NADPH Oxidases/SGLT2 Pro-Oxidant Pathway
Abstract
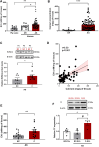
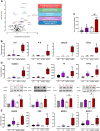
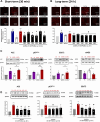
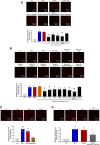
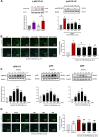
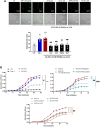
Pathological tissues release a variety of factors, including extracellular vesicles (EVs) shed by activated or apoptotic cells. EVs trapped within the native pathological valves may act as key mediators of valve thrombosis. Human aortic stenosis EVs promote activation of valvular endothelial cells, leading to endothelial dysfunction, and proadhesive and procoagulant responses.
Keywords: SGLT2i; SVD; TAVI; TAVR; aortic stenosis; extracellular vesicles; inflammation; leaflet; microparticles; thrombosis.
© 2024 The Authors.
Conflict of interest statement
This work was supported by an unrestricted research grant by the Groupe pour l’Enseignement de la Recherche Cardio-vasculaire en Alsace, France. The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures



References
-
- Goody P.R., Hosen M.R., Christmann D., et al. Aortic valve stenosis. Arterioscler Thromb Vasc Biol. 2020;40(4):885–900. - PubMed
-
- Trimaille A., Hmadeh S., Matsushita K., Marchandot B., Kauffenstein G., Morel O. Aortic stenosis and the hemostatic system. Cardiovasc Res. 2023;119(6):1310–1323. - PubMed
-
- Sellers S.L., Turner C.T., Sathananthan J., et al. Transcatheter aortic heart valves: histological analysis providing insight to leaflet thickening and structural valve degeneration. JACC Cardiovasc Imaging. 2019;12(1):135–145. - PubMed
-
- Hein M., Schoechlin S., Schulz U., et al. Long-term follow-up of hypoattenuated leaflet thickening after transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2022;15(11):1113–1122. - PubMed
LinkOut - more resources
Full Text Sources

