Colloidal spherical stibnite particles via high-temperature metallo-organic synthesis
- PMID: 39170978
- PMCID: PMC11334972
- DOI: 10.1039/d4na00020j
Colloidal spherical stibnite particles via high-temperature metallo-organic synthesis
Abstract
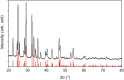
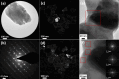
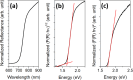
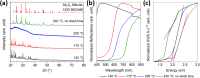
Antimony trisulfide (Sb2S3) is an emerging semiconductor with a high absorption coefficient and a bandgap in the visible range. This makes it a promising material for various electronic and optoelectronic applications. However, one of the main challenges is still the synthesis of the material, as it is usually obtained either as a nanomaterial in its amorphous form with inferior optical properties or in crystalline rod-like structures in the micrometer or sub-micrometer range, which leads to application-related difficulties such as clogging in inkjet printing or spraying processes or highly porous layers in film applications. In this study, a one-pot synthesis of highly crystalline, spherical Sb2S3 sub-micron particles is presented. The particles are growing encapsulated in a removable, wax-like matrix that is formed together with an intermediate from the precursors SbCl3 and l-cysteine. Both substances are insoluble in the reaction mixture but well-dispersable in the solvent 1-octadecene (ODE). The intermediate forms a complex crosslinked architecture whose basic building block consists of an Sb atom attached to three cysteine molecules via Sb-S bonds. Embedded in the matrix consisting of excess cysteine, ODE, and chlorine, the intermediate decomposes into amorphous Sb2S3 particles that crystallize as the reaction proceeds at 240 °C. The final particles are highly crystalline, spherical, and in the sub-micron range (420 ± 100 nm), making them ideal for further processing. The encapsulation method could not only provide a way to extend the size range of colloidal particles, but in the case of Sb2S3, this method circumvents the risk of carbonization of ligands or insufficient crystallization during the annealing of amorphous material.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


References
-
- Gruber H. The learning curve in the production of semiconductor memory chips. Appl. Econ. 1992;24:885–894. doi: 10.1080/00036849200000056. - DOI
-
- Wang L. Zhao J. Liu H. Huang J. Design, modification and application of semiconductor photocatalysts. J. Taiwan Inst. Chem. Eng. 2018;93:590–602. doi: 10.1016/j.jtice.2018.09.004. - DOI
LinkOut - more resources
Full Text Sources

