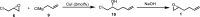Synthetic Process Development of (R)-(+)-1,2-Epoxy-5-hexene: An Important Chiral Building Block
- PMID: 39171130
- PMCID: PMC11334172
- DOI: 10.1021/acs.oprd.4c00101
Synthetic Process Development of (R)-(+)-1,2-Epoxy-5-hexene: An Important Chiral Building Block
Abstract
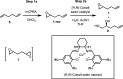
Herein, we describe two practical approaches to synthesize (R)-(+)-1,2-epoxy-5-hexene from inexpensive and readily available raw materials and reagents. The first approach is a two-step sequence, involving an epoxidation with meta-chloroperoxybenzoic acid (mCPBA) and a chiral resolution with (salen)Co(II), producing (R)-(+)-1,2-epoxy-5-hexene in 24-30% overall yield. The second approach utilizes readily available (R)-epichlorohydrin as the starting material and features an epoxide ring-opening reaction with allylMgCl and the NaOH-mediated ring closure reaction. Development of this two-step process affords R-(+)-1,2-epoxy-5-hexene in overall isolated yields of 55-60% with an exceptional purity profile. Both approaches have been successfully demonstrated on 100-200 g scales.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- Cleator E.; Sheen F. J.; Bio M. M.; Jos Brands K. M.; Davies A. J.; Dolling U.-H. Regioselective Synthesis of N-Substituted-4-Substituted Isothiazolidine-1,1-Dioxides. Tetrahedron Lett. 2006, 47 (25), 4245–4248. 10.1016/j.tetlet.2006.04.038. - DOI
-
- Cleator E.; Baxter C. A.; O’Hagan M.; O’Riordan T. J. C.; Sheen F. J.; Stewart G. W. Synthesis of Novel Benzoxathiazepine-1,1-Dioxides by Means of a One-Pot Multicomponent Reaction. Tetrahedron Lett. 2010, 51 (7), 1079–1082. 10.1016/j.tetlet.2009.12.099. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources