High-fat and high-carbohydrate diets increase bone fragility through TGF-β-dependent control of osteocyte function
- PMID: 39171528
- PMCID: PMC11343608
- DOI: 10.1172/jci.insight.175103
High-fat and high-carbohydrate diets increase bone fragility through TGF-β-dependent control of osteocyte function
Abstract
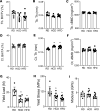
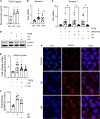
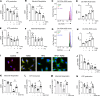
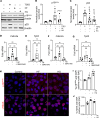
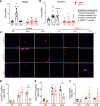
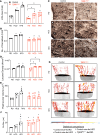
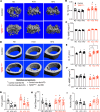
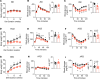
Obesity can increase the risk of bone fragility, even when bone mass is intact. This fragility stems from poor bone quality, potentially caused by deficiencies in bone matrix material properties. However, cellular and molecular mechanisms leading to obesity-related bone fragility are not fully understood. Using male mouse models of obesity, we discovered TGF-β signaling plays a critical role in mediating the effects of obesity on bone. High-carbohydrate and high-fat diets increase TGF-β signaling in osteocytes, which impairs their mitochondrial function, increases cellular senescence, and compromises perilacunar/canalicular remodeling and bone quality. By specifically inhibiting TGF-β signaling in mouse osteocytes, some of the negative effects of high-fat and high-carbohydrate diets on bones, including the lacunocanalicular network, perilacunar/canalicular remodeling, senescence, and mechanical properties such as yield stress, were mitigated. DMP1-Cre-mediated deletion of TGF-β receptor II also blunted adverse effects of high-fat and high-carbohydrate diets on energy balance and metabolism. These findings suggest osteocytes are key in controlling bone quality in response to high-fat and high-carbohydrate diets. Calibrating osteocyte function could mitigate bone fragility associated with metabolic diseases while reestablishing energy balance.
Keywords: Bone biology; Obesity.
Conflict of interest statement
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

