Tetraphenylpentalenide organolanthanide complexes
- PMID: 39171542
- PMCID: PMC11513172
- DOI: 10.1039/d4cc02570a
Tetraphenylpentalenide organolanthanide complexes
Abstract
The D2h symmetrical 1,3,4,6-tetraphenylpentalenide is an excellent ligand for the stabilisation of strongly coloured bis(pentalenide) LnIII sandwich complexes. These easily accessible compounds complement previously reported lanthanide organometallics and provide new opportunities to understand the roles of the f-orbitals in electronic structure and bonding.
Conflict of interest statement
Figures
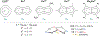
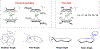
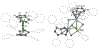


References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

